- EN - English
- CN - 中文
Immunofluorescence of GFAP and TNF-α in the Mouse Hypothalamus
GFAP和TNF-α在小鼠下丘脑的免疫荧光
发布: 2021年07月05日第11卷第13期 DOI: 10.21769/BioProtoc.4078 浏览次数: 6052
评审: Xiaoyi ZhengGisela HelferHe Liu

相关实验方案
高灵敏且可调控的 ATOM 荧光生物传感器:用于检测细胞中蛋白质靶点的亚细胞定位
Harsimranjit Sekhon [...] Stewart N. Loh
2025年03月20日 2243 阅读
Abstract
Immunofluorescence is a reliable method for identifying specific proteins in neuronal and glial cell populations of the hypothalamus. Several immunofluorescence protocols are available to detect protein markers and neuropeptides in the hypothalamus; however, published methods may vary in subtle details that can potentially impact the final outcome of the procedure. Here, we provide a detailed protocol suitable for thin cryostat sections, which has been successful for specific antibodies directed against key markers of hypothalamic neurons and glial cells. We include every detail concerning brain tissue collection, processing, sectioning, and labeling with optimal dilutions of antibodies with the aim of reducing non-specific background. Our background-optimized immunostaining protocol has been routinely used in the lab and allows efficient detection of specific neuropeptides, glial cells, and markers of inflammation and endoplasmic reticulum stress in the hypothalamus.
Keywords: Hypothalamus (下丘脑)Background
Elucidating the protein organization in neurons and glia is essential for understanding hypothalamic function. Immunofluorescence is a powerful tool to investigate changes in the expression of neuronal and glial proteins and to map activated brain areas at the cellular level. This imaging technique enables the visualization of highly specific protein (antigen) targets using a combination of primary and secondary antibodies. Antigenic determinants of interest in brain tissue are first treated with a monoclonal or polyclonal primary antibody, targeted toward peptides from the species/protein of interest generated in a host (mouse-, rabbit-, chicken-, rat-, donkey-derived, etc., are available), followed by a secondary antibody directed toward the host of the primary antibody and conjugated to a synthetic or natural fluorophore. The antibody targets often labeled in brain tissue sections are glial fibrillary acidic protein (GFAP), neuronal and axonal neurofilaments, neuropeptides, microtubule-associated proteins, blood-brain barrier proteins, pre- and post-synaptic proteins, myelin, markers of inflammation, and numerous antigens associated with specific diseases. Among the useful fluorescent markers for the visualization of antigens in brain tissue are rhodamine, fluorescein, the Alexa Fluor series, and the cyanine dyes. Counterstaining for nuclei using a variety of popular DNA-binding dyes (such as TO-PRO-3 iodide) follows treatment with the secondary antibody.
This method has been previously used by our laboratory to identify neuronal subpopulations in various hypothalamic nuclei involved in energy homeostasis (Dalvi et al., 2017). In particular, using double immunofluorescence staining, we identified GFAP in the hypothalamus and tumor necrosis factor (TNF)-α, a marker of inflammation, in the hypothalamic arcuate nucleus following acute lipid infusion and chronic high-fat diet feeding in mice, which may contribute to a significant alteration in content or expression of appetite-regulating neuropeptides. To the best of our knowledge, this was the first description of TNF-α induction demonstrated by immunofluorescence in the mouse hypothalamic arcuate nucleus after exposure to a high-fat diet. This method can be applied to the identification of other markers of inflammation and endoplasmic reticulum (ER) stress in the hypothalamic regions provided that reliable antibodies are available for detection. This protocol details a generalized procedure for staining frozen brain cryosections ranging from 20 to 30 µm in thickness. Figures 1 and 2 presented in this protocol are confocal images that reveal the expression of GFAP and TNF-α in the hypothalamic arcuate nucleus in 25 µM brain sections of mice treated acutely with lipid or chronically with a high-fat diet (Dalvi et al., 2017).
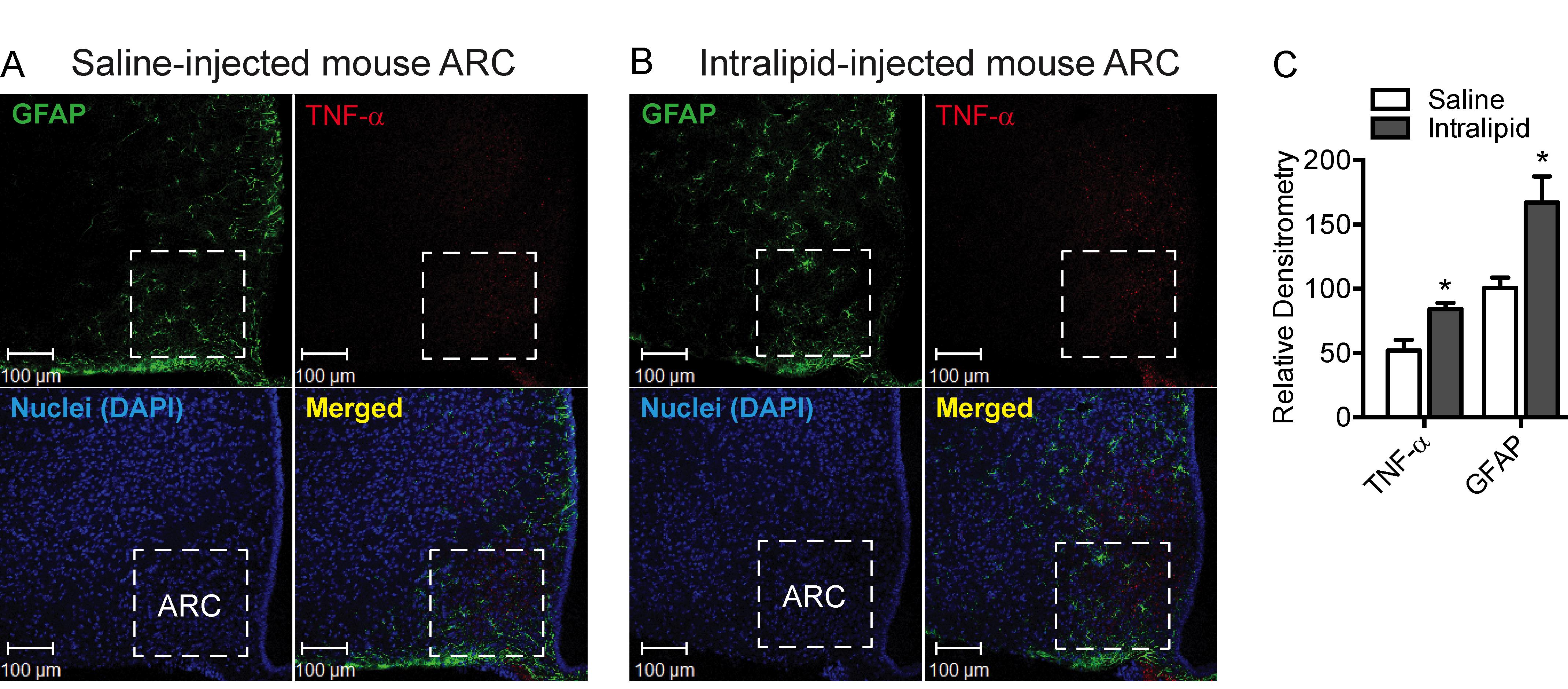
Figure 1. Effect of acute intralipid treatment at 24 h post-infusion in C57BL/6 mice on hypothalamic arcuate nucleus (ARC) reactive astrocytosis and expression of TNF-α, a marker of inflammation. A-B.Representative images showing immunofluorescence for the expression of GFAP (green) and TNF-α (red) in the hypothalamic ARC of C57BL6 mice at 24 h following acute infusion with saline (A) or Intralipid (B). C. Quantification of the immunofluorescence intensity for GFAP and TNF-α expression in the ARC of C57BL/6 mice at 24 h following acute infusion with saline or Intralipid. Data in the bar graph are expressed as the mean ± SEM (n = 5-6 animals/group); *P < 0.05 versus control. Images in A and B and the graph in C are adapted and modified from Dalvi et al. (2017).
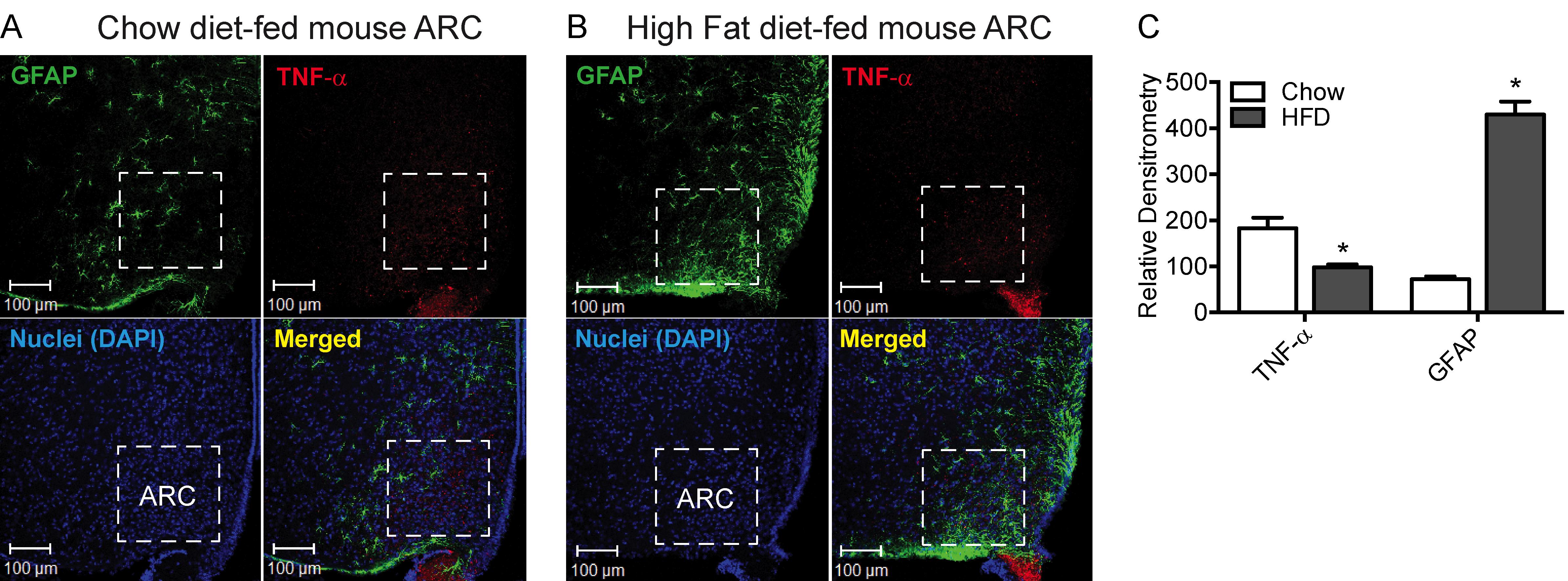
Figure 2. Effect of high-fat diet (HFD, 60% kcal) feeding for 8 weeks in CD-1 mice on reactive astrocytosis and expression of TNF-α in the hypothalamic arcuate nucleus (ARC). A-B. Representative images showing immunofluorescence for the expression of GFAP (green) and TNF-α (red) in the hypothalamic ARC of CD-1 mice fed chow (A) or an HFD (B) for 8 weeks. C. Quantification of immunofluorescence intensity of GFAP and TNF-α expression in the ARC of CD-1 mice fed either chow or an HFD for 8 weeks. Data in the bar graph are expressed as the mean ± SEM (n = 5-6 animals/group); *P < 0.05 versus control. Images in A and B and the graph in C are adapted and modified from Dalvi et al. (2017).
Materials and Reagents
50 ml Falcon tubes
Aluminum foil
Fine paint brushes
Cryostat blades (Leica Biosystems, catalog number: 3802106-TRI-FACET/LOPRO/CTD Blade-10PK)
12-well plates to store frozen sections (BD Falcon, Corning, catalog number: 353043)
24-well plates to store frozen sections (BD Falcon, Corning, catalog number: 353047)
Coverslips (25 × 50 mm) (VWR International, catalog number: 48393-059)
SuperFrost Plus slides (positively charged) (Thermo Fisher Scientific, catalog number: 12-550-15)
Wax pen (PAP Pen) (Abcam, catalog number: ab2601)
Fresh 4% paraformaldehyde in PBS (75 ml per mouse) (Millipore Sigma, catalog number: 158127-100G)
0.9% saline (Millipore Sigma, catalog number: S8776-100ML)
PBS pH 7.4 (Thermo Fisher Scientific, Gibco, catalog number: 10010023)
15% and 30% sucrose in PBS (300 g sucrose in 1 L PBS can be stored in the fridge for up to a month) (Millipore Sigma, catalog number: S7903-1KG)
Cryoprotectant solution (Millipore Sigma, catalog number: G7893-500M)
Tissue embedding solution (Tissue-Tek® O.C.T. compound) (Sakura® Finetek, VWR, catalog number: 25608-930)
Tween 20 for preparation of PBS-T (0.06% Tween-20 in PBS) (Millipore Sigma, catalog number: P1379-500ML)
Triton X-100 for preparation of PBS-Triton-X100 (0.4% Triton-X100 in PBS) (Millipore Sigma, catalog number: X100-500ML)
Normal donkey serum for preparation of blocking buffer (5% serum in PBS-Triton-X100) (Abcam, catalog number: ab7475)
Normal donkey serum for preparation of antibody buffer (2% serum in PBS-Triton-X100) (Abcam, catalog number: ab7475)
Nuclear stain: TOPRO (TO-PRO-3 iodide 642/661) (Life Technologies, catalog number: T3605)
Pro-Long Gold anti-fade mountant (mounting medium) (Thermo Fisher Scientific, catalog number: P10144)
Clear nail polish (or equivalent for sealing coverslips)
Primary antibodies:
Anti-GFAP: Chicken-derived polyclonal mouse antibody against GFAP (1:500 dilution) (Abcam, catalog number: ab4674)
Anti-TNF-α: Goat-derived polyclonal mouse antibody against TNF-α (1:50 dilution) (Santa Cruz, catalog number: SC-1350)
Secondary antibodies (fluorescently tagged secondary antibody against species from which primary antibodies are obtained):
Donkey anti-Chicken IgY (H+L) Secondary Antibody, FITC (will interact with the GFAP chicken-derived anti-mouse primary antibody) (Thermo Fisher Scientific, Invitrogen, catalog number: SA1-72000)
Donkey anti-Goat IgG (H+L) Cross-Adsorbed Secondary Antibody, Alexa Fluor 568 (will interact with the TNF-α goat-derived anti-mouse primary antibody) (Thermo Fisher Scientific, Invitrogen, catalog number: A-11057)
Equipment
Scalpel
Tweezers
Fine scissors
Spatula
-20°C freezer
Adult Mouse Brain Slicer Matrix (Zivic Instruments, catalog number: BSMAS001-1)
Mouse perfusion apparatus (VWR, Leica Biosystems, catalog number: 10030-382)
Cryostat (Leica, model: CM3050S or similar)
Confocal laser scanning microscope ((Zeiss, model: LSM 510 or similar)
Software
AxioVision 3.0 imaging software (Carl Zeiss GmbH, Jena, Germany)
ImageJ software, National Institutes of Health, Bethesda, MD, USA (Schneider et al., 2012)
GraphPad Prism software, Inc., La Jolla, CA, USA
Procedure
文章信息
版权信息
© 2021 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Dalvi, P. S. and Belsham, D. D. (2021). Immunofluorescence of GFAP and TNF-α in the Mouse Hypothalamus. Bio-protocol 11(13): e4078. DOI: 10.21769/BioProtoc.4078.
分类
神经科学 > 细胞机理 > 小神经胶质
生物化学 > 蛋白质 > 荧光
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link