- EN - English
- CN - 中文
Visualization of Host Cell Kinase Activation by Viral Proteins Using GFP Fluorescence Complementation and Immunofluorescence Microscopy
利用GFP荧光互补和免疫荧光显微镜观察病毒蛋白对宿主细胞激酶的激活
(*contributed equally to this work) 发布: 2021年07月05日第11卷第13期 DOI: 10.21769/BioProtoc.4068 浏览次数: 5043
评审: Anonymous reviewer(s)

相关实验方案

诱导型HIV-1库削减检测(HIVRRA):用于评估外周血单个核细胞中HIV-1潜伏库清除策略毒性与效力的快速敏感方法
Jade Jansen [...] Neeltje A. Kootstra
2025年07月20日 2460 阅读
Abstract
Non-receptor protein-tyrosine kinases regulate cellular responses to many external signals and are important drug discovery targets for cancer and infectious diseases. While many assays exist for the assessment of kinase activity in vitro, methods that report changes in tyrosine kinase activity in single cells have the potential to provide information about kinase responses at the cell population level. In this protocol, we combined bimolecular fluorescence complementation (BiFC), an established method for the assessment of protein-protein interactions, and immunofluorescence staining with phosphospecific antibodies to characterize changes in host cell tyrosine kinase activity in the presence of an HIV-1 virulence factor, Nef. Specifically, two Tec family kinases (Itk and Btk) as well as Nef were fused to complementary, non-fluorescent fragments of the Venus variant of YFP. Each kinase was expressed in 293T cells in the presence or absence of Nef and immunostained for protein expression and activity with anti-phosphotyrosine (pTyr) antibodies. Multi-color confocal microscopy revealed the interaction of Nef with each kinase (BiFC), kinase activity, and kinase protein expression. Strong BiFC signals were observed when Nef was co-expressed with both Itk and Btk, indicative of interaction, and a strong anti-pTyr immunoreactivity was also seen. The BiFC, pTyr, and kinase expression signals co-localized to the plasma membrane, consistent with Nef-mediated kinase activation in this subcellular compartment. Image analysis allowed calculation of pTyr-to-kinase protein ratios, which showed a range of responses in individual cells across the population that shifted upward in the presence of Nef and back down in the presence of a kinase inhibitor. This method has the potential to reveal changes in steady-state non-receptor tyrosine kinase activity and subcellular localization in a cell population in response to other protein-kinase interactions, information that is not attainable from immunoblotting or other in vitro methods.
Keywords: Protein-tyrosine kinase (蛋白酪氨酸激酶)Background
Non-receptor protein-tyrosine kinases, exemplified by members of the Src and Tec kinase families, regulate many aspects of cell biology including growth, differentiation, and motility in response to diverse stimuli (Amatya et al., 2019). Methods that assess the spatial and temporal aspects of tyrosine kinase signaling at the single cell and population levels are essential to better understanding their function. In this protocol, we provide details of a cell-based method to evaluate protein-protein interaction, kinase activity, and subcellular localization of Tec family kinases in response to the interaction with the HIV-1 accessory protein, Nef. This approach is potentially applicable to many other kinase systems in which protein-protein interactions impact kinase activity.
Nef is a small (27-34 kDa, depending on the subtype) membrane-associated protein unique to the primate lentiviruses HIV-1, HIV-2, and SIV (Foster and Garcia, 2008). HIV-1 Nef enhances viral infectivity, supports high-titer replication in vivo, and promotes immune escape of HIV-infected cells (Basmaciogullari and Pizzato, 2014; Pawlak and Dikeakos, 2015). Rhesus macaques infected with nef-defective SIV exhibit very low viral loads and do not progress to simian AIDS (Kestler et al., 1991), illustrating an essential role for Nef in viral pathogenesis. Along the same lines, individuals infected with nef-defective HIV-1 can remain AIDS-free in the absence of antiretroviral therapy for many years (Deacon et al., 1995; Kirchhoff et al., 1995).
Nef lacks intrinsic biochemical activities, functioning instead through interactions with host cell proteins related primarily to endocytic trafficking and kinase signaling pathways (Staudt et al., 2020).Nef hijacks non-receptor tyrosine kinases of the Src and Tec families normally linked to immune receptor activation to enhance HIV-1 replication. Nef directly activates the Src family members Hck and Lyn by binding to their SH3 domains (Briggs et al., 1997; Trible et al., 2006). Selective inhibition of Nef-mediated Src family kinase activation blocks Nef-dependent enhancement of HIV-1 infectivity and replication (Emert-Sedlak et al., 2009 and 2013). Tec family kinases play essential roles in B- and T-cell receptor signaling (Andreotti et al., 2010), with the interleukin-2 inducible T-cell kinase (Itk) and Bruton's tyrosine kinase (Btk) expressed in primary HIV-1 target cells (CD4+ T cells and macrophages, respectively). Readinger et al. provided the first evidence linking Itk to HIV-1 entry, viral transcription, assembly, and release (Readinger et al., 2008). A subsequent study showed that Nef provides a link between HIV-1 infection and Tec family kinase signaling by demonstrating direct interaction between Nef and both Itk and Btk at the cell membrane (Tarafdar et al., 2014). Treatment of HIV-infected T cells with a selective Itk inhibitor blocked Nef-dependent enhancement of viral infectivity and replication. Importantly, activation of both Src and Tec family kinases is highly conserved across all M group HIV-1 subtypes, consistent with an important function in the HIV-1 life cycle and viral pathogenesis (Narute and Smithgall, 2012; Emert-Sedlak et al., 2013; Tarafdar et al., 2014). For an in-depth review of Nef interactions with host cell tyrosine kinases, see Staudt et al. (2020).
In a recent study, we explored the molecular mechanisms of Tec family kinase activation by the Nef proteins of HIV-1 and SIV (Li et al., 2020). By combining cell-based bimolecular fluorescence complementation (BiFC) (Romei and Boxer, 2019) and anti-phosphotyrosine immunofluorescence microscopy, we found that HIV-1 Nef interacts with Itk and Btk at the cell membrane and results in constitutive kinase activity. For the BiFC assay, Itk or Btk and Nef were fused to complementary, non-fluorescent fragments of the Venus variant (Nagai et al., 2002) of YFP. The kinases were then expressed in 293T cells either alone or together with Nef, followed by immunostaining for Nef and kinase protein expression as well as protein-tyrosine phosphorylation using anti-phosphotyrosine (pTyr) antibodies. Multi-color confocal microscopy enabled simultaneous assessment of Nef-kinase complex formation (BiFC), kinase activity (anti-pTyr immunofluorescence), and kinase protein expression (anti-kinase immunofluorescence). When expressed alone, both Itk and Btk showed a diffuse subcellular staining pattern with the anti-kinase antibody and weak reactivity with the anti-pTyr antibody. In contrast, co-expression with Nef induced a strong BiFC signal with both Itk and Btk, indicative of interaction, and a strong anti-pTyr immunoreactivity was seen. The BiFC, pTyr, and kinase expression signals co-localized to the cell membrane, consistent with Nef-mediated kinase activation in this subcellular compartment. As a control, cells were treated with Tec family kinase inhibitors (Lin et al., 2004; Roskoski, 2016) that suppressed the pTyr signal but did not affect BiFC, demonstrating that interaction of Nef with Tec family kinases at the membrane does not require kinase activity.
Results were quantitated at the single-cell level using the NIH ImageJ image analysis software (Schneider et al., 2012). Kinase expression and tyrosine phosphorylation immunofluorescence signal intensities for at least 100 cells for each condition were expressed as the mean pTyr-to-kinase protein fluorescence intensity ratios. This analysis enabled statistical comparisons of cell populations and showed that cells co-expressing Itk or Btk and Nef had significantly higher fluorescence ratios as compared with those expressing the kinase alone or the inhibitor-treated cells expressing the Nef-kinase complex.
Using the same approach, we also investigated Nef-stimulated Itk and Btk autophosphorylation on their respective activation loop tyrosine residues (pTyr511 and pTyr551, respectively), a step required for Tec family kinase activation (Joseph et al., 2013). Cells were transfected with the Nef and Itk/Btk Venus fusion constructs for BiFC as before, but in this case the cells were stained with phosphospecific antibodies for the activation loop phosphotyrosines in place of the general anti-pTyr antibody. Co-expression with Nef led to significant increases in Itk and Btk activation loop autophosphorylation, which also localized almost exclusively to the cell membrane. SIV Nef (mac239 allele) was also found to strongly induce membrane-associated autophosphorylation of both kinases, demonstrating that Tec family kinase activation is conserved across Nef proteins from diverse primate lentiviruses. A representative confocal image from this study is shown in Figure 1.
While the experimental approach described above was developed to explore kinase interaction with, and activation by, a viral protein in a cell-based setting, the overall concept should be readily adaptable to any combination of kinases and interacting partners. The cell-based approach has important advantages over older in vitro methods such as immune-complex kinase assays, which do not provide information about the subcellular localization of the active kinase complex or the range of responses across a population of individual cells. However, one important caveat includes consideration of where to add the fragments of Venus for the BiFC assay. For example, both Nef and Tec family kinases localize to the cell membrane by virtue of N-terminal signals. Nef is myristoylated on its N-terminus, while Tec kinases have an N-terminal Pleckstrin homology (PH) domain that binds to membrane phosphoinositides. To avoid interference with these membrane-targeting signals, we were careful to fuse the Venus fragments to the C-terminus of each protein. Control experiments are also essential to verify that Venus fragment fusion does not influence basal kinase activity or localization, which is readily accomplished by comparing unfused with fused versions of each kinase in transfected cells and staining with kinase and phosphospecific antibodies. Finally, it should be noted that the Venus fluorophore, once reconstituted via BiFC, is irreversible. While this feature of BiFC may help to stabilize transient interactions for endpoint assessment by microscopy as described here, other techniques are more appropriate to assess the kinetics of interaction, such as the split-FAST reversible complementation system (Tebo and Gautier, 2019).
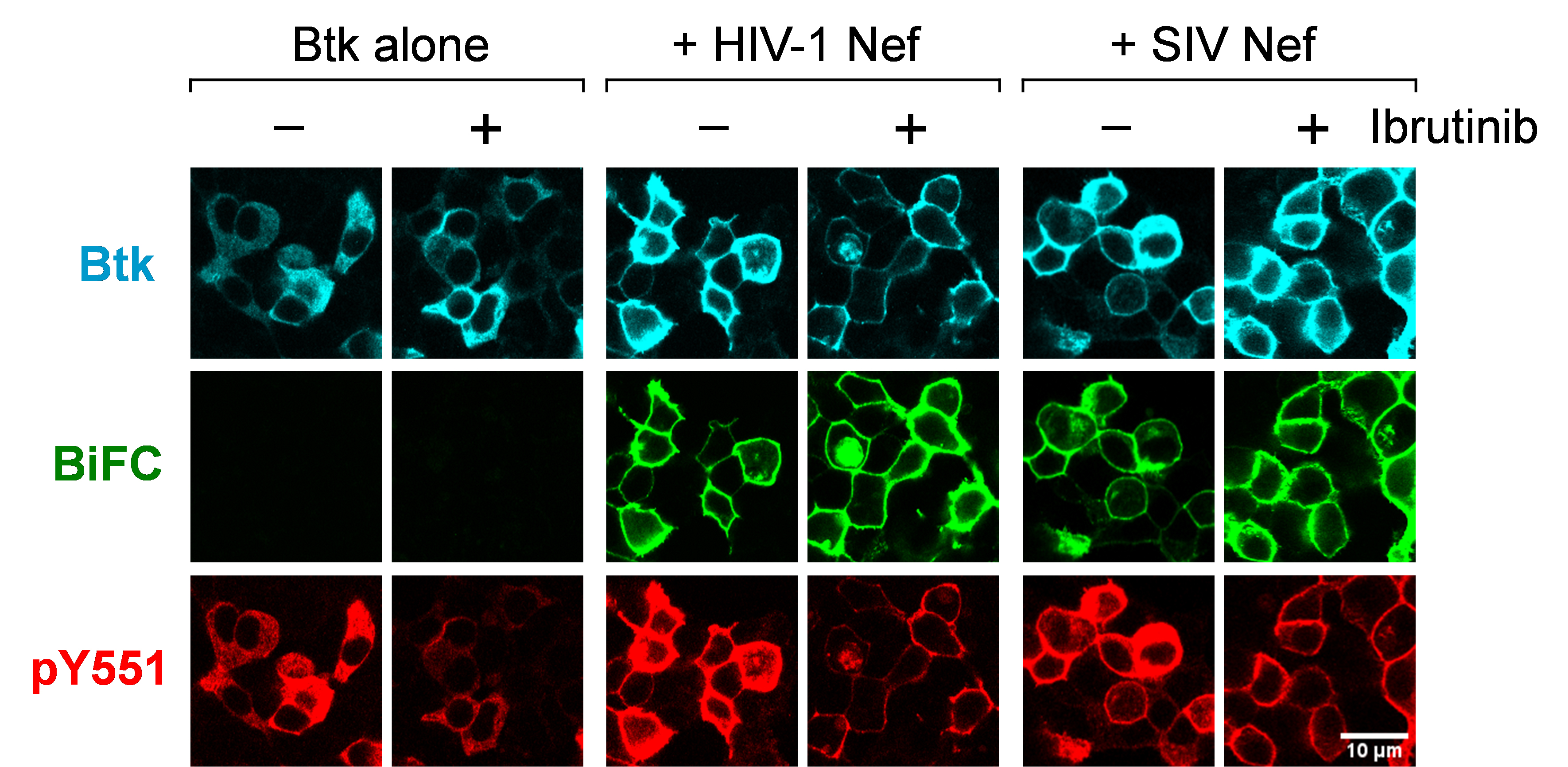
Figure 1. HIV-1 and SIV Nef proteins induce constitutive Btk activation loop autophosphorylation at the cell membrane. Btk was expressed in 293T cells either alone or together with HIV-1 Nef (SF2 isolate) or SIV Nef (mac239) as BiFC pairs in the absence or presence of the Btk/Itk inhibitor ibrutinib (1 μM). Cells were fixed and stained for confocal microscopy with phosphospecific antibodies against the Btk activation loop phosphotyrosine (pY551; red) and the Btk protein (V5 epitope; cyan). Nef interaction with Btk is observed as fluorescence complementation of the YFP variant, Venus (BiFC; green). Note that interaction and kinase activation occur at the plasma membrane.
Materials and Reagents
Molecular biology reagents
Phusion high-fidelity DNA polymerase (New England Biolabs, catalog number: M0530S)
Venus template (gift from Dr. Atsushi Miyawaki, RIKEN Brain Science Institute, Saitama, Japan)
HIV-1 (SF2 allele) and SIV (mac239) Nef clones (NIH AIDS Reagent Program, HIV #11431; SIV #2476)
Full-length human Tec family kinase cDNA clones (Dana-Farber/Harvard Cancer Center PlasmID DNA Resource Core, Btk # HsCD00346954; Itk # HsCD00021352)
Mammalian expression vector, pCDNA3.1(−) (Thermo Fisher, catalog number: V79520)
Anti-V5 tag mouse monoclonal antibody (Thermo Fisher, catalog number: R960-25)
Anti-V5 tag rabbit polyclonal antibody (Sigma, catalog number: AB3792)
BTK anti-pY551 rabbit monoclonal antibody (Abcam, catalog number: ab40770)
Anti-pTyr antibody pY99 (Santa Cruz, catalog number: sc-7020)
Anti-HIV-1 Nef monoclonal antibody 6.2 (NIH AIDS Reagent Program, catalog number: 1539)
Goat anti-rabbit IgG (H+L), mouse/human ads-TXRD (Texas Red conjugate; cross-adsorbed to mouse and human immunoglobulins; Southern Biotech, catalog number: 4050-07)
Goat anti-mouse IgG (H+L), human ads-TXRD (Texas Red conjugate; cross-adsorbed to human immunoglobulins; Southern Biotech, catalog number: 1031-07)
Pacific Blue goat anti-mouse IgG antibody (Thermo Fisher/Molecular Probes, catalog number: P31582)
Pacific Blue goat anti-rabbit IgG antibody (Thermo Fisher/Molecular Probes, catalog number: P10994)
35 mm microwell dishes (MatTek, catalog number: P35G-1.5-14-C)
Human embryonic kidney 293T cells (American Type Culture Collection, catalog number: CRL-11268)
Dulbecco’s modified Eagle’s medium (DMEM; ThermoFisher/Invitrogen, catalog number: 11965-118)
Fetal bovine serum (FBS; Gemini Bio-Products, catalog number: 900-108)
Trypsin-EDTA, 0.05% (ThermoFisher/Invitrogen catalog number: 25300054)
X-tremeGENE 9 DNA transfection reagent (Sigma-Aldrich, catalog number: 06365787001)
Paraformaldehyde, 16% aqueous solution (Fisher, catalog number: 50980487)
Triton X-100 (Sigma, catalog number: X100-1L)
Bovine serum albumin (BSA, Sigma, catalog number: A3059-500G)
Itk inhibitor, BMS-509744 (Calbiochem, catalog number: 41-982-05MG)
Itk/Btk inhibitor, ibrutinib (SelleckChem, catalog number: S2680)
Equipment
Olympus FluoView FV1000 Confocal Microscope
Software
Prism v. 8.0 (GraphPad Software, Inc.; www.graphpad.com)
ImageJ (National Institutes of Health; https://imagej.net/Welcome)
Olympus FluoView Software (https://www.olympus-lifescience.com/en/)
Procedure
文章信息
版权信息
© 2021 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Shu, S. T., Li, W. F. and Smithgall, T. E. (2021). Visualization of Host Cell Kinase Activation by Viral Proteins Using GFP Fluorescence Complementation and Immunofluorescence Microscopy. Bio-protocol 11(13): e4068. DOI: 10.21769/BioProtoc.4068.
- Li, W. F., Aryal, M., Shu, S. T. and Smithgall, T. E. (2020). HIV-1 Nef dimers short-circuit immune receptor signaling by activating Tec-family kinases at the host cell membrane. J Biol Chem 295(15): 5163-5174.
分类
生物化学 > 蛋白质 > 成像
微生物学 > 微生物-宿主相互作用 > 病毒
微生物学 > 微生物信号传导 > 磷酸化
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link










