- EN - English
- CN - 中文
Operant Vapor Self-administration in Mice
小鼠蒸汽吸入自我给药操作
发布: 2021年05月20日第11卷第10期 DOI: 10.21769/BioProtoc.4023 浏览次数: 4569
评审: Miao HeXing LiuAnonymous reviewer(s)
Abstract
Models of drug addiction in rodents are instrumental in understanding the underlying neurobiology. Intravenous self-administration of drugs in mice is currently the most commonly used model; however, several challenges exist due to complications related to catheter patency. To take full advantage of the genetic tools available to study opioid addiction in mice, we developed a non-invasive mouse model of opioid self-administration using vaporized fentanyl. This model can be used to study various aspects of opioid addiction including self-administration, escalation of drug intake, extinction, reinstatement, and drug seeking despite adversity. Further, this model bypasses the limitations of intravenous self-administration and allows the investigation of drug taking over extended periods of time and in conjunction with cutting-edge techniques such as calcium imaging and in vivo electrophysiology.
Keywords: Vapor (蒸气)Background
Vapor inhalation is emerging as an alternative route of drug self-administration (instead of intravenous) to study addiction to opioids and other drugs. This has been accomplished in rats using alcohol (Vendruscolo and Roberts, 2014; de Guglielmo et al., 2017), nicotine (Smith et al., 2020), cannabis (Freels et al., 2020; Muthusamy, 2020), and opioids such as sufentanil (Vendruscolo et al., 2018), fentanyl (McConnell et al., 2020), and heroin (Gutierrez et al., 2020). We recently adapted this model and developed a mouse model of opioid vapor self-administration using fentanyl (Moussawi et al., 2020), the protocol for which is described in this manuscript.
Protocol overview: Mice (males and females) are trained to self-administer fentanyl vapor for 1-h sessions, during which each response on the active operandum (active lever press or nosepoke into the active port) triggers a vapor delivery on a fixed-ratio 1 (FR1) schedule of reinforcement (this can be adjusted to higher FRs, e.g., 3, 5, or 10) (Video 1). Each vapor delivery is followed by a timeout period of 60 s, during which additional responses on the operandum are recorded but do not result in additional drug delivery. The timeout period is signaled by a cue light that is turned on and remains on for the duration of the timeout. Operant activity on an inactive lever/nosepoke port is recorded but has no consequences. After reaching stable pressing (<25% variation in the number of vapor deliveries relative to the average vapor deliveries of the 3 preceding sessions; usually 5-8 sessions, at least 24-h apart), mice are matched based on their operant response and split between short- (ShA) and long-access (LgA) groups. ShA mice are allowed to self-administer fentanyl vapor for 1 h every other day, whereas LgA mice are allowed to self-administer fentanyl for 6 or 12 h every other day. Escalation of fentanyl intake is typically observed over 8-10 sessions in the LgA group, whereas response in the ShA group remains stable.
Video 1. Video showing a mouse self-administering fentanyl vapor. Note the increased locomotion caused by fentanyl intake and the straub tail reaction after vapor delivery. Reprinted/adapted from Moussawi et al. (2020). © The Authors, some rights reserved; exclusive licensee, American Association for the Advancement of Science. Distributed under a Creative Commons Attribution NonCommercial License 4.0 (CC BY-NC) http://creativecommons.org/licenses/by-nc/4.0/
Materials and Reagents
Animals
Adult (male and female, 8-10 weeks old) C57BL/6J mice are purchased from JAX Laboratories (Bar Harbor, ME, USA). The mice are allowed to habituate in the animal facility for a week. Mice are group-housed and maintained on a 12 h reverse light/dark cycle in a room with a controlled temperature (22 ± 2°C) and humidity (50%). Mice have free access to water and food in their home cages. Training sessions are performed during the dark cycle, and the body weight is recorded at least once a week.
Fentanyl citrate (NIDA Drug Supply Program, Bethesda, MD, USA)
Vegetable glycerin (Essential Elements, Boulder, CO, USA, available on Amazon; UPC code: 703610139756)
Propylene glycol (MP Biochemicals, catalog number: 151957)
Capsaicin (AK Scientific, catalog number: N735-5g)
Ethanol 200 proof (Pharmco-Aaper, catalog number: 111000200)
Narcan kit (available at pharmacies)
Equipment
Chambers
Airtight chambers are customized to order and made from transparent plexiglass (Figure 1) (La Jolla Alcohol Research, La Jolla, CA, USA). These can be nosepoke or lever press chambers.
Nosepoke chambers
Two nosepoke ports (1 cm in diameter) are mounted opposite to each other on the side walls, 1.5 cm from the floor. White light bulbs are mounted inside the nosepoke ports. The vapor delivery port is mounted in one of the walls or in the door of the chamber, 7 cm from the chamber floor. The exhaust (suction) port is mounted in one of the walls, preferably opposite the vapor delivery port, 12 cm from the chamber floor.
Note: You can substitute nosepoke chambers for lever chambers: the inner and outer dimensions are the same. The levers and cue lights are installed in the right and left walls. The levers are positioned 1.5 cm from the floor, and the cue lights 6.5 cm from the floor. The vapor delivery and exhaust ports are positioned similarly to the nosepoke chambers.
Chamber enclosure
The chambers are placed in a black Plexiglas enclosure to minimize noise and light. The enclosures can fit four or eight individual chambers, depending on the size of the enclosure.
Vaporizer: SVS250 vaporizer (Scientific Vapor, OR, USA)
Drug solution tanks: TFV8 X-baby Smok tanks, 4 ml (Shenzhen IVPS Technology, catalog number: TC005301000)
Coils for the tanks: V8 X-Baby Q2, 0.4 Ω dual coils (Shenzhen IVPS Technology, catalog number: TA104301000)
Exhaust system
The goal of this system is to generate negative pressure in the operant chamber, which drives vapor into the chamber and then clears it out: an air compressor pump (75 DG model HK-25L; Hakko, catalog number: E307612) that generates vacuum suction is connected to the chamber on one end, and to an inline disposable HEPA-Cap filter (Whatman 6702-3600 hepa-Cap 36, Cole Parmer, catalog number: EW-29700-92) on the other end (Figure 1B). The tubing from the HEPA filter is then connected to the facility’s exhaust system.
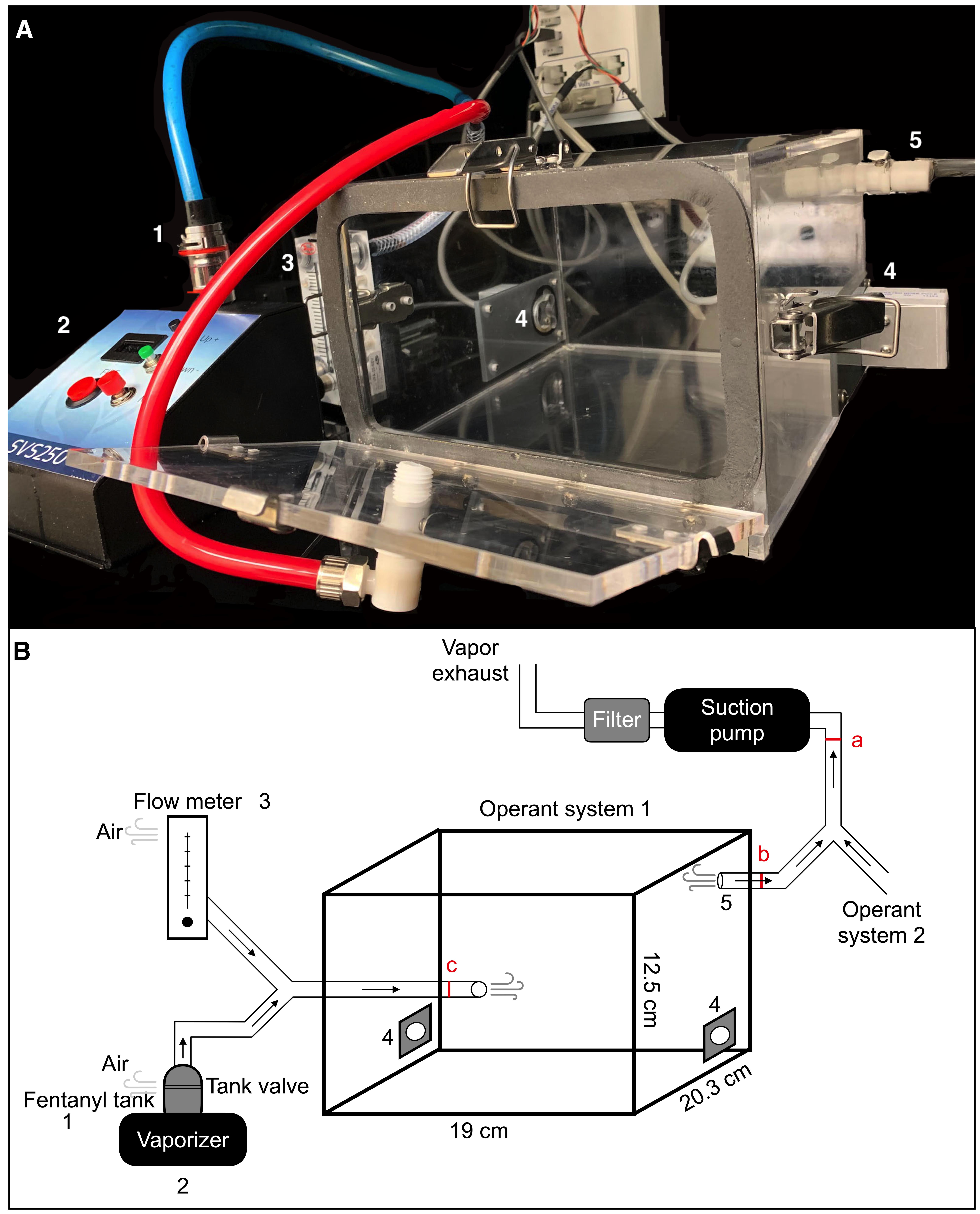
Figure 1. Vapor chamber setup. A. Different components of the vapor chamber apparatus: (1) tank where the fentanyl solution is loaded; (2) vaporizer; (3) flow meter; (4) nosepoke ports on opposite walls; and (5) exhaust port that connects to the vacuum suction pump. Photo credit: Maria Ortiz, NIDA. B. Sketch of the vapor chamber setup including airflow dynamics, suction pump, and HEPA filter. The numbers in B correspond to those in A. Inner dimensions of the vapor chamber are indicated in cm; the internal volume of the chamber is ~5 L. The pump creates negative pressure that determines the airflow rate in the chambers. The total negative pressure generated by the pump is measured by connecting a flow meter between the pump and the tubing from all the chambers – red bar a (~16 L/min). We measure the total airflow in each chamber by connecting a flow meter between the exhaust port and the tubing from the pump – red bar b (4 L/min). To check whether the chamber is airtight, we measure the airflow between the inlet port and the tubing from the vaporizer – red bar c; if this does not match the total airflow from the chamber measured near the exhaust port (at red bar b), the chamber is not airtight and the leak must be addressed. Reprinted/adapted from Moussawi et al. (2020). © The Authors, some rights reserved; exclusive licensee, American Association for the Advancement of Science. Distributed under a Creative Commons Attribution NonCommercial License 4.0 (CC BY-NC) http://creativecommons.org/licenses/by-nc/4.0/.Air flow controller (Dwyer, model: VFA-23-SSV)
Med-associates hardware (Med Associates, Fairfax, VT, USA):
DIG-700G (decode card – PCI)
DIG-704PCI-2 (interface card – PCI)
SG-6080D (small tabletop cabinet + power supply (120 V/60 Hz))
SG-210CB (DB-25 smart control cable, M/F, 25' (7.6 m)
DIG-716 (SmartCtrlTM interface module, 4 in/8 out)
SG-716 (SmartCtrlTM connection panel, 4 in/8 out)
Software
MedPC (Med Associates, Fairfax, VT, USA)
GraphPad Prism (Version 8, GraphPad Software, San Diego, CA, USA)
Procedure
文章信息
版权信息
© 2021 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Marchette, R. C. N., Tunstall, B. J., Vendruscolo, L. F. and Moussawi, K. (2021). Operant Vapor Self-administration in Mice. Bio-protocol 11(10): e4023. DOI: 10.21769/BioProtoc.4023.
- Moussawi, K., Ortiz, M. M., Gantz, S. C., Tunstall, B. J., Marchette, R. C. N., Bonci, A., Koob, G. F., and Vendruscolo, L. F. (2020). Fentanyl vapor self-administration model in mice to study opioid addiction. Sci Adv 6(32): eabc0413.
分类
神经科学 > 神经系统疾病 > 动物模型
神经科学 > 行为神经科学 > 实验动物模型 > 小鼠
生物科学
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link













