- EN - English
- CN - 中文
In vitro and In vivo CD8+ T Cell Suppression Assays
CD8+ T细胞体外和体内抑制试验
(*contributed equally to this work) 发布: 2021年05月20日第11卷第10期 DOI: 10.21769/BioProtoc.4020 浏览次数: 7095
评审: Luis Alberto Sánchez VargasMarieta RusevaAnonymous reviewer(s)

相关实验方案

基于人外周血单个核细胞(PBMCs)和浆细胞样树突细胞(pDCs)的宿主靶向抗病毒药物(HTA)筛选方案
Zhao Xuan Low [...] Pouya Hassandarvish
2025年03月05日 3068 阅读

用于比较人冷冻保存 PBMC 与全血中 JAK/STAT 信号通路的双磷酸化 CyTOF 流程
Ilyssa E. Ramos [...] James M. Cherry
2025年11月20日 2351 阅读
Abstract
CD8+CD28− T suppressor cells (Ts) have been documented to promote immune tolerance by suppressing effector T cell responses to alloantigens following transplantation. The suppressive function of T cells has been defined as the inhibitory effect of Ts on the proliferation rate of effector T cells. 3H-thymidine is a classical immunological technique for assaying T cell proliferation but this approach has drawbacks such as the inconvenience of working with radioactive materials. Labeling T cells with CFSE allows relatively easy tracking of generations of proliferated cells. In this report, we utilized antigen presenting cells (APCs) and T cells matched for human leukocyte antigen (HLA) class I or class II to study CD8+CD28- T cell suppression generated in vitro by this novel approach of combining allogeneic APCs and γc cytokines. The expanded CD8+CD28- T cells were isolated (purity 95%) and evaluated for their suppressive capacity in mixed lymphocyte reactions using CD4+ T cells as responders. Here, we present our adapted protocol for assaying the Ts allospecific suppression of CFSE-labeled responder T cells.
Keywords: CD8+CD28- T cells (CD8+CD28-T 细胞)Background
T regulatory cells (Tregs) with dedicated suppressor function play a crucial role in the homeostatic control of immunity in the periphery. Regulatory CD8+ T cells have also been demonstrated to play an important role in neonatal tolerance and autoimmune diseases (Tang et al., 2005). There are two broad categories of immune regulation by Tregs: non-specific and antigen-specific. Non-specific immunosuppression potentially causes general immunosuppression and produces undesirable side effects, such as infectious diseases. These Tregs include CD8+CD25+, CD8+CD122+, CD45RClow, and IL-2/GM-CSF-induced CD8+ Tregs. On the contrary, antigen-specific Tregs are primed during the immune response to foreign or self-antigens and subsequently specifically downregulate that immune response. These Tregs include CD8+CD28-, CD8+CD75s+, plasmacytoid dendritic cell (DC2)-induced CD8+, CD8+CD45RChigh Tc1, and TCR peptide-specific CD8αα Tregs. CD8+CD28− Tregs have been recently documented to play an important role in alloimmunity. In our previous studies, we have expanded large numbers of human CD8+CD28− Tregs in a relatively short period of time by stimulating CD8+ T cells with APCs following supplementation with the triple common gamma chain cytokines IL-2, IL-7, and IL-15 in vitro; however, the detailed characteristics of the expanded CD8+CD28− Tregs were unclear. Moreover, the principal function of this population when transferred in vivo was yet to be examined. Measurement of suppression has been achieved through the co-culture of Tregs and T effector cells. Methods include the detection of cell proliferation, cytokine production, and activation markers (CD25 or CD134) (Long et al., 2017). CFSE-based co-culture has become the gold standard for proliferation assays and has been used successfully to assess the function of Tregs. Here, based on CFSE co-culture assays, we show that the in vitro-expanded CD8+CD28− Tregs maintain allospecific suppressive capacity both in vitro (Figure 1) and in vivo.
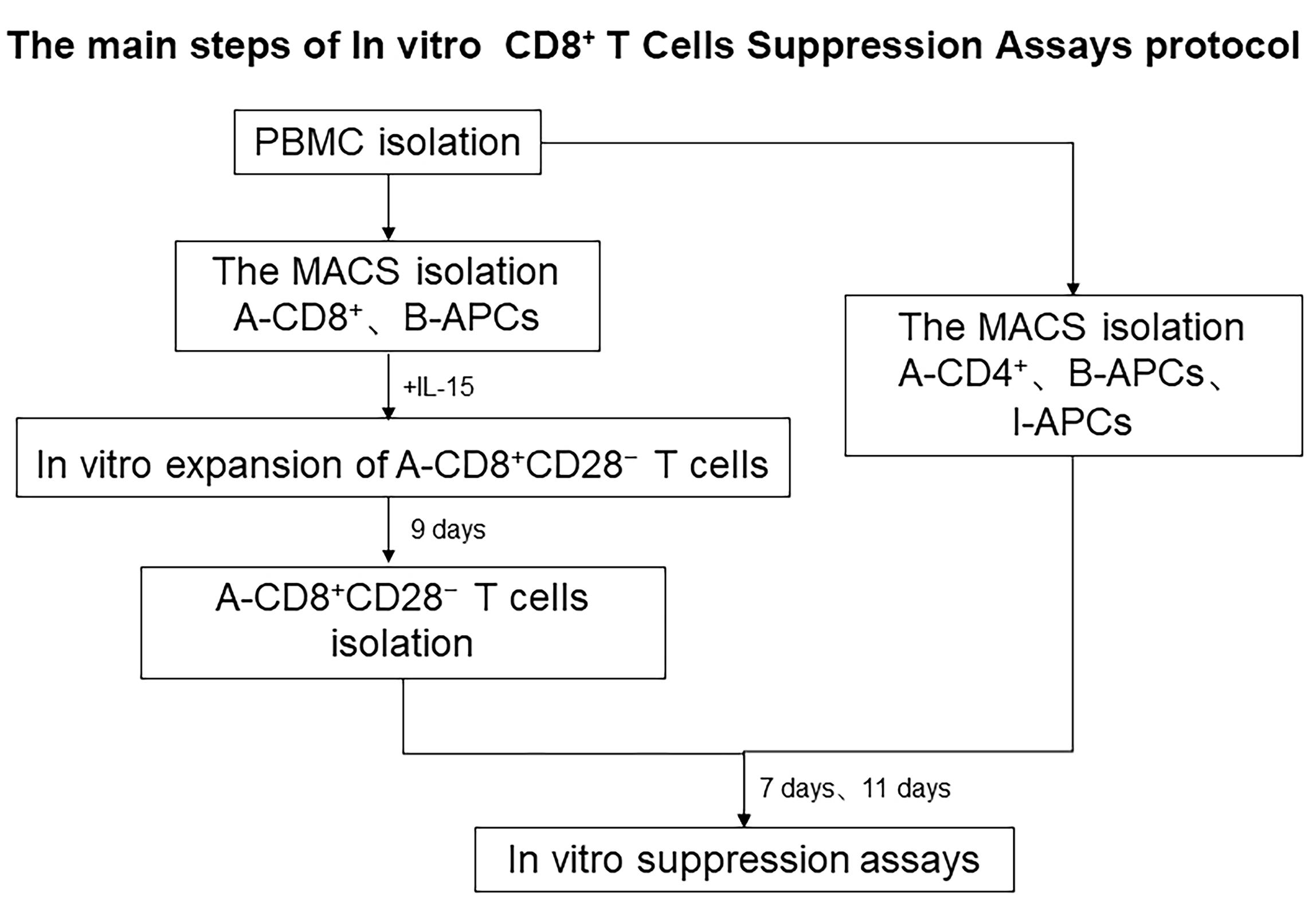
Figure 1. The main steps of the in vitro CD8+ T cell suppression assay protocol
Materials and Reagents
Heparin sodium anticoagulation tubes, 5 ml (JiangSu, YuLi)
Centrifuge tubes, 50 ml (Corning, catalog number: 430829)
Centrifuge tubes, 15 ml (Corning, catalog number: 430791)
24-well round-bottomed plates (Corning, catalog number: 3524)
96-well round-bottomed plates (Corning, catalog number: 3799)
MidiMACS separator (Miltenyi, catalog number: 130-042-302)
LS column (Miltenyi, catalog number: 130-042-401)
Disposable syringe with a needle (ShuangGe, China)
70-μm cell strainer (Biologix, catalog number: 15-1070)
NOG mice (Beijing Vital River Laboratory Animal Technology Co Charles River Laboratories)
Ficoll-Hypaque solution (Haoyang Biologiacal, TBD sciences, catalog number: LTS1077)
RPMI 1640, 500 ml (ThermoFisher, Gibco, catalog number: C11875500BT)
FBS Qualified Australia Origin (ThermoFisher, Gibco, catalog number: 10099141 C)
Bovine serum albumin, BSA (SIJIA, catalog number: N0008-1)
Phosphate-buffered saline, PBS (ThermoFisher, Gibco, catalog number: C10010500BT)
CD8 MicroBeads, human (Miltenyi, catalog number: 130-045-201)
CD28 MicroBeads, human (Miltenyi, catalog number: 130-093-247)
CD2 MicroBeads, human (Miltenyi, catalog number: 130-091-114)
CD4 MicroBeads, human (Miltenyi, catalog number: 130-045-101)
IL-2 (PeproTech, catalog number: AF-200-02-50)
IL-7 (PeproTech, catalog number: AF-200-07-50)
IL-15 (PeproTech, catalog number: AF-200-15-50)
10× RBC lysis buffer (ThermoFisher, Invitrogen, catalog number: 00-4300-54)
Trypan Blue solution 0.4%, liquid (MERCK,Sigma-Aldrich, catalog number: T8154-100ML)
7-AAD viability stain solution (ThermoFisher, Invitrogen, catalog number: 00-6993-50)
CFDA, SE (ThermoFisher, Invitrogen, catalog number: C1157)
Flow cytometry antibodies
AlexaFluorTM 700 mouse anti-human CD3 monoclonal antibody, OKT3 (ThermoFisher, eBioscience, catalog number: 56-0037-42)
efluor 450 mouse anti-human CD8a monoclonal antibody, SK1 (ThermoFisher, eBioscience, catalog number: 48-0088-41)
APC-mouse anti-human CD28 monoclonal antibody, CD28.2 (ThermoFisher, eBioscience, catalog number: 17-0289-42)
APC-mouse anti-human CD4 monoclonal antibody, OKT4 (ThermoFisher,eBioscience, catalog number: 17-0048-42)
FITC mouse anti-human CD2 monoclonal antibody, RPA-2.10 (ThermoFisher, eBioscience, catalog number: 11-0029-42)
PE-mouse anti-human CD45 monoclonal antibody, HI30 (ThermoFisher,eBioscience, catalog number: 12-0459-42)
Fixable viability stain (FVS) 620 100 μg (BD Pharmingen, catalog number: 564996)
NaCl
KCl
Na2HPO4
KH2PO4
EDTA
1× PBS (pH 7.4) (see Recipes)
D-PBS (pH 7.4) (see Recipes)
1% BSA-PBS (see Recipes)
0.5% BSA-PBS (see Recipes)
Equipment
Centrifuge (Eppendorf, model: 5810R)
Electronic balance (Sartorius, model: BP61)
Microelectronic balance (OHAUS, model: AX124ZH)
Hemocytometer
Constant temperature water box
Incubator (Thermo, model: Thermo3111)
FACS LSRFortessa (BD)
Finnpipette (Eppendorf)
Magnetic stirrer (BG-stirrelDB)
Optical microscope (CONIC, XDS-1B)
Clean bench
Software
Flowjo vX.0.7
SPSS 20.0
GraphPad Prism 5.01
Procedure
文章信息
版权信息
© 2021 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Xie, L., Liu, G., Liu, Y. and Yu, Y. (2021). In vitro and In vivo CD8+ T Cell Suppression Assays. Bio-protocol 11(10): e4020. DOI: 10.21769/BioProtoc.4020.
分类
免疫学 > 免疫细胞功能 > 抗原特异反应
免疫学 > 免疫细胞功能 > 细胞因子
细胞生物学 > 基于细胞的分析方法 > 细胞间的相互作用
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link










