- EN - English
- CN - 中文
Identification of R-loop-forming Sequences in Drosophila melanogaster Embryos and Tissue Culture Cells Using DRIP-seq
DRIP-seq法鉴定黑腹果蝇胚胎和组织培养细胞R-loop形成序列
发布: 2021年05月05日第11卷第9期 DOI: 10.21769/BioProtoc.4011 浏览次数: 7145
评审: Qin TangAnonymous reviewer(s)
Abstract
R-loops are non-canonical nucleic structures composed of an RNA–DNA hybrid and a displaced ssDNA. Originally identified as a source of genomic instability, R-loops have been shown over the last decade to be involved in the targeting of proteins and to be associated with different histone modifications, suggesting a regulatory function. In addition, R-loops have been demonstrated to form differentially during the development of different tissues in plants and to be associated with diseases in mammals. Here, we provide a single-strand DRIP-seq protocol to identify R-loop-forming sequences in Drosophila melanogaster embryos and tissue culture cells. This protocol differs from earlier DRIP protocols in the fragmentation step. Sonication, unlike restriction enzymes, generates a homogeneous and highly reproducible nucleic acid fragment pool. In addition, it allows the use of this protocol in any organism with minimal optimization. This protocol integrates several steps from published protocols to identify R-loop-forming sequences with high stringency, suitable for de novo characterization.
Graphic abstract:
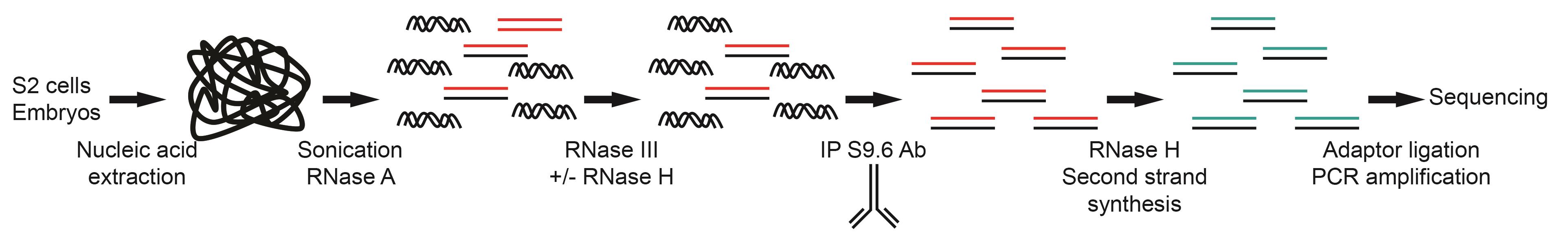
Figure 1. Overview of the strand-specific DRIP-seq protocol
Background
Overview
R-loops are triple-stranded nucleic acid structures that form when an RNA hybridizes with a complementary ssDNA, leading to displacement of the second DNA strand. R-loops were first described as a by-product of transcription and a source of genomic instability that needed to be resolved. However, research over the last decade has shown that R-loops can be associated with specific histone modifications and transcriptional status, induce the targeting of proteins, or act as promoters (Chedin and Benham, 2020; Niehrs and Luke, 2020). To study R-loop function, protocols have been developed to map these structures in multiple organisms. Differences have been observed between species: R-loop-forming sequences in mammals have a GC skew; those in Saccharomyces cerevisiae have an AT skew; and in plants, both GC and AT skews have been reported (Sanz et al., 2016; Wahba et al., 2016; Xu et al., 2017; Hartono et al., 2018). Differences in the properties and localization of R-loop-forming sequences between species and the association of R-loops with different diseases and gene misexpression highlight the importance of developing standard R-loop mapping protocols to perform rigorous comparisons.
R-loop maps in plants have shown that some R-loops can form differentially during development (Fang et al., 2019; Xu et al., 2020) and may potentially be involved in changes in transcriptional status. In mammals, active enhancers have been associated with R-loop formation, and these R-loops can act as promoters (Tan-Wong et al., 2019). The study of R-loops in the context of development in intact organisms with available genetic tools to evaluate their formation, resolution, and function, will allow a better understanding of R-loop biology.
Here, we provide a detailed strand-specific DRIP-seq protocol to identify R-loop-forming sequences in Drosophila melanogaster embryos and tissue culture cells (Figure 1). Similar to other DRIP protocols, our protocol relies on the S9.6 antibody to identify R-loop-forming sequences but also considers that the weak affinity of the S9.6 antibody for dsRNA can generate false positive signals (Phillips et al., 2013; Hartono et al., 2018; König et al., 2017). This single-strand DRIP-seq protocol in association with the genetic tools available for Drosophila melanogaster should be a powerful system to study R-loop function in the whole organism, during development, and in different tissues (Figure 2, Table 1). DRIP-seq can be combined with genetic manipulation to overexpress or knockdown genes with a view to identifying their effect on R-loop formation, or with cell synchronization to follow R-loop formation during the cell cycle. Finally, the use of sonication rather than restriction enzymes to fragment the genomic DNA means that this protocol could potentially be adapted to other organisms with minimal optimization.
Limitations
The protocol relies on the S9.6 antibody to immunoprecipitate RNA–DNA hybrid-containing fragments. This antibody is not a perfect tool: it shows differential binding affinity based on the sequence of the R-loop with no correlation with the GC content and a preference for longer RNA–DNA hybrids. It can bind dsRNA, albeit with an affinity 5-times lower than that for RNA–DNA hybrids (Phillips et al., 2013; König et al., 2017). The latter two limitations can be overcome during the preparation of nucleic acids. After nucleic acid extraction, ssRNA and dsRNA are removed by incubating the nucleic acids with RNase A in the presence of 0.5 M NaCl to avoid the degradation of RNA in the RNA–DNA hybrids. This first digestion step is followed by an incubation with RNase III to degrade any dsRNA that were not degraded by RNase A. By using two different RNases before immunoprecipitation, we removed all ssRNA and dsRNA but also potentially some R-loops, which can be sensitive to these RNases. Thus, these steps make the experiment more stringent and limit false positives but could be modified to balance stringency with sensitivity, since some R-loops may be sensitive to these treatments. Removal of RNA that can interact with S9.6 is most relevant when the RNA component of the R-loop is to be sequenced; although, we have observed that the RNA–DNA hybrid pulldown efficiency is increased when stringent RNase treatment is used. It is also essential to have an RNase H-treated negative control to evaluate the specificity of the pulldown (Figure 3). We found that commercially available E. coli RNase H does not consistently completely digest RNA–DNA hybrids in nucleic acid preparations. This can be solved by producing and using highly active human RNase H1 and RNase H2. This negative control is especially important during optimization of the DRIP protocol; it ensures that the technique is specific. It is also used to call peaks when DRIP-seq is performed (Figures 2, 4, and 5).
To avoid bias toward longer fragments during the immunoprecipitation step, we fragment the nucleic acids by sonication (Figure 4A). Sonication allows us to obtain a homogenous population of fragments with an average size of 300 bp. Sonication is performed after RNase A treatment to avoid the possibility of generating new RNA–DNA hybrids during the DRIP procedure. Sonication is reported to reduce the recovery of some RNA–DNA hybrids as compared with restriction digestion (Crossley et al., 2020).
Advantages
Advantages compared with other DRIP protocols
This protocol uses a gentle lysis step to extract the nucleic acids. Cell lysis can be performed on tissue culture cells, whole embryos, or dissected tissues from larval, pupal, or adult Drosophila melanogaster. Although the ~200 mg Drosophila embryos needed to perform one DRIP-seq experiment is relatively high, the possibility to freeze tissues and pool them for a single nucleic acid extraction makes it feasible. By dissecting Drosophila melanogaster and performing DRIP on discs, organs, or sorted cell populations, it should be possible to identify cell- or tissue-specific R-loops.
This DRIP protocol, contrary to several protocols developed for mammalian cells (e.g., Sanz et al., 2016), does not use a restriction enzyme cocktail to fragment the nucleic acids. Instead, sonication is used, which leads to the fragmentation of nucleic acids at an average size of 300 bp (Figure 4A). These fragments have a homogenous size and the sonication is highly reproducible. An increase in resolution by using sonication, as compared with restriction digestion, in mammalian cells has recently been demonstrated (Crossley et al., 2020). By using sonication, the protocol can easily be adapted to other organisms with little optimization; the only step that may require optimization is lysis. Sonication has another advantage: it leads to disruption of the displaced single-strand DNA of the R-loop, which makes it possible to prepare strand-specific sequencing libraries using either the DNA or the RNA moiety of the RNA–DNA hybrid (Wahba et al., 2016).
Advantages compared with other methods
Another method to identify R-loop-forming sequences relies on a catalytically inactive form of the RNase H1 enzyme (dRNase H1) (Ginno et al., 2012; Chen et al., 2017). The use of dRNase H1 in cells presents several potential problems, which may explain the differences in the identification of R-loop-forming sequences with this method versus the S9.6 antibody. Firstly, RNase H1 is not the only regulator of R-loops in cells; it has been suggested that topoisomerases are the main enzymes responsible for the resolution of R-loops that form co-transcriptionally in human cells (Manzo et al., 2018; Zhang et al., 2019), while RNase H1 and H2 target R-loops once they are formed. Secondly, a recent article by Lockhart et al., 2019 demonstrated that RNase H1 is activated upon stress, while RNase H2 displays the main activity under physiological conditions in S. cerevisiae. Thirdly, with dRNase H1, R-loop identification may be limited to R-loops that are accessible, protein-free, and normally degraded by RNase H enzymes. Fourthly, expression of dRNase H1 may stabilize R-loops. While this may allow detection of transient R-loops, it could also skew interpretation of where stable R-loops exist. Finally, RNase H1 has two RNA–DNA hybrid binding domains, both of which need to bind to induce degradation of the RNA moiety of the hybrid (Nowotny et al., 2008). This may prevent or limit the detection of smaller RNA–DNA hybrids. Thus, while dRNase H1 may be a useful tool in some contexts, the interpretation of DRIP results may be more straightforward. Comparison of results from both methods could also yield complementary information.
Similar to DRIP, dRNase H1 has been used to isolate RNA–DNA hybrids after extraction of nucleic acids from tissue culture cells; however, bias of the enzyme toward longer hybrids and its weaker affinity as compared with the S9.6 antibody make it less efficient (Ginno et al., 2012).
Finally, native bisulfite sequencing is an alternative high-resolution method for identifying R-loops. Bisulfite converts cytosine to uracil in single-stranded DNA (Yu et al., 2003); thus, this method does not detect RNA–DNA hybrids but the ssDNA strand that is displaced when they form. However, the presence of methyl-cytosine in the genome blocks modification of the ssDNA, and ssDNA can be displaced by the formation of other non-canonical DNA structures such as G-quadruplexes and I-motifs, which could lead to false negative or false positive results, respectively.
The single-strand DRIP-seq protocol presented here has been optimized to identify R-loop-forming sequences in Drosophila melanogaster embryos and tissue culture cells, and can potentially be used in other organisms with minimal optimization. This protocol could be a standardized means to evaluate R-loops across developmental stages or in different tissues or organisms.
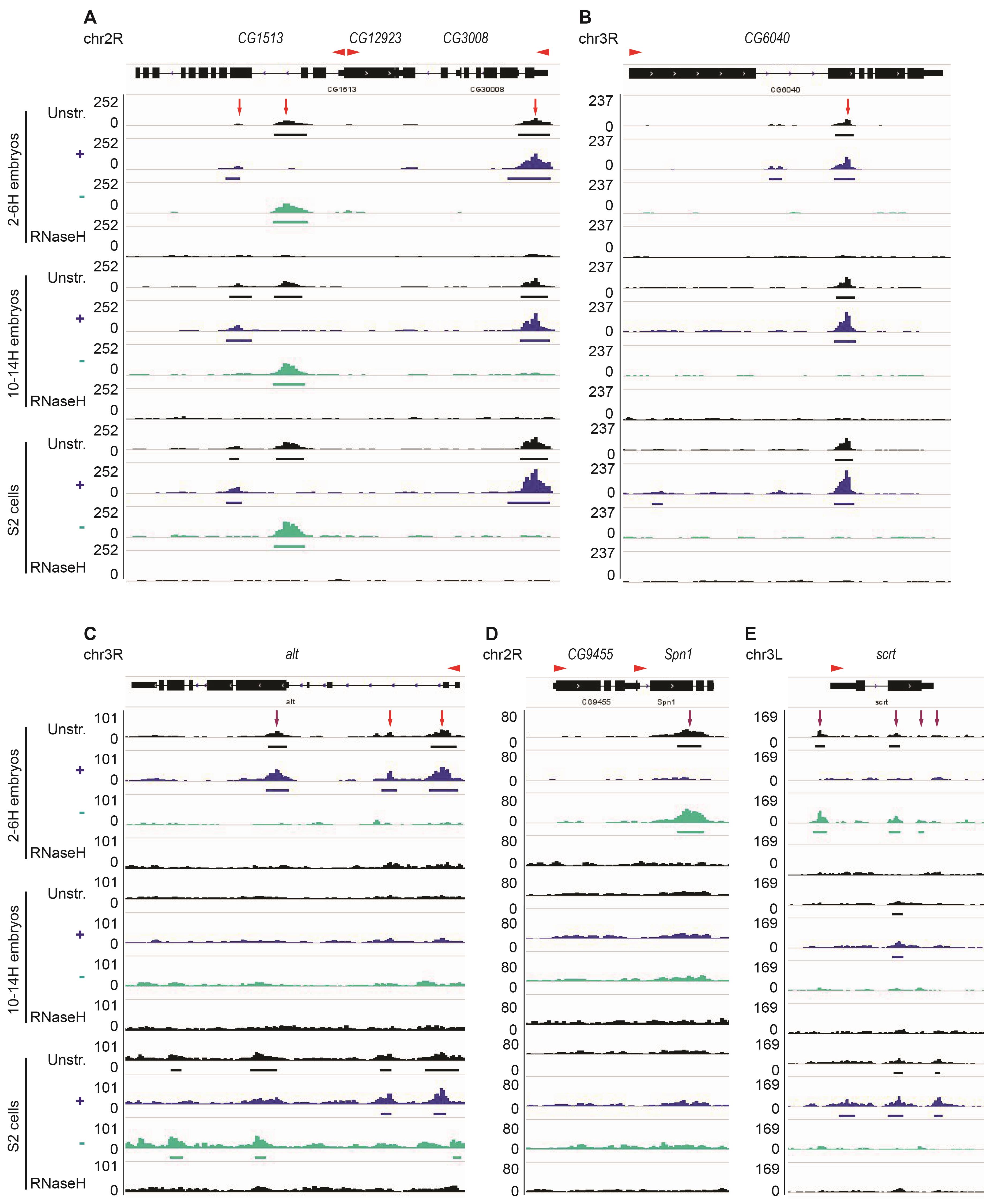
Figure 2. Examples of shared and distinct R-loops among S2 cells and early and later stage embryos. A-B. R-loops form over the CG1513, CG3008, and CG6040 genes in S2 cells and 2-6 H and 10-14 H embryos (red arrow). No R-loop is formed over the CG12923 gene. C. At the alt gene, three R-loops are detected in 2-6 H but not in 10-14 H embryos. Two of these R-loops (red arrows) are also present in S2 cells, while one of them is replaced by an R-loop on the opposite strand (purple arrow). D. R-loop formed over Spn1 is only present in 2-6 H embryos (purple arrows). E. R-loops form over scrt on the + strand in 2-6 H embryos and the - strand in 10-14 H embryos and in S2 cells. “Unstr” corresponds to unstranded DRIP-seq data, “+” and “-” indicate the strand-specific track, and RNase H-treated sample acts as a negative control. The red arrowhead indicates the orientation of the transcript. Note that the “+” and “-” strands refer to the DNA strand such that “+” strand R-loops should arise from transcription in the leftward direction. Thus, in A, the R-loop in the CG3008 gene is in the expected orientation to have arisen from gene transcription, while that in the gene CG1513 would be derived from antisense transcription.
Table 1. Data availability
Materials and Reagents
RNase H1 and H2 expression and purification
Amicon® ultra centrifugal filter, 0.5 ml 10K (EMD Millipore, catalog number: UFC5010BK)
Amicon® ultra centrifugal filter, 0.5 ml 3K (EMD Millipore, catalog number: UFC5003BK)
Econo-column (2.5*20 cm) (Bio-Rad, catalog number: 7374252)
E. coli RosettaTM 2(DE3)pLysS SinglesTM Competent Cells – Novagen (Sigma, catalog number: 71401)
Ni-NTA agarose (Qiagen, catalog number: 1018244)
Glutathione-superflow resin (Takara, catalog number: 635607)
PreScission protease plus (Homemade; commercial enzyme could also be used)
Ampicillin (Bioshop, catalog number: AMP201.100)
LB Broth Miller (Bioshop, catalog number: LBL407.500)
Albumin, bovine serum (Bioshop, catalog number: ALB001.100)
Lysozyme (Bioshop, catalog number: LYS702.5)
Imidazole (OmniPur) (Millipore, catalog number: 5710-OP)
Glycerol (Bioshop, catalog number: GLY001.4)
IPTG (Bioshop, catalog number: IPT002.5)
RNase H2 extraction buffer (see Recipes)
RNase H1 lysis buffer (see Recipes)
RNase H1 wash buffer (see Recipes)
RNase H1 elution buffer (see Recipes)
RNase H size column buffer (see Recipes)
RNase H storage buffer (see Recipes)
Protease inhibitors (see Recipes)
Protease inhibitors and additives
TLCK (Sigma, catalog number: T7254)
Benzamidine (Bioshop, catalog number: BEN601.25)
Pepstatin A (Bioshop, catalog number: PEP605.25)
1,10-Phenanthroline (Sigma, catalog number: 131377-5G)
PMSF (Fisher Scientific, catalog number: 19538125)
Aprotinin (Bioshop, catalog number: APR200)
Leupeptin (Bioshop, catalog number: LEU011.50)
NP40 (Nonidet P40 substitute, Fluka catalog number: 74385) (can substitute Sigma, catalog number: 74385)
DTT (Bioshop, catalog number: DTT002.100)
Activity testing of RNase H1 and H2
rNTP (NEB, catalog number: N0450S)
UTP α-P32 (Perkin Elmer, catalog number: BLU007H250UC)
T7 RNA polymerase (NEB, catalog number: M0251L)
SSC 20× (see Recipes)
Tri-sodium citrate (Bioshop, catalog number: CIT001.205)
Drosophila melanogaster embryo collection
Fly bottles
Oregon R flies (Dr Éric Lécuyer lab; available through Bloomington Drosophila stock center)
Agar A (Bioshop, catalog number: FB0010)
Sugar (RedPath)
Apple juice
TEGOSEPT, 1 KG (Nipagin) (Diamed.ca, catalog number: GEN20-258)
Homemade sieve made with NITEX (can be obtained from https://flystuff.com/)
Funnel
Fly cages (fly cages, food, and bottles can be obtained from https://flystuff.com/)
Fly food (prepared in-house)
Methanol (Bioshop, catalog number: MET302.4)
Embryo lysis buffer (see Recipes)
Apple juice plate (see Recipes)
1× PBS (see Recipes)
1× PBT (see Recipes)
Cell culture
S2 cells (Invitrogen, catalog number: R69007)
GibcoTM Schneider's Drosophila Sterile Medium (Thermo Fisher Scientific, catalog number: 21720-024)
FBS (Thermo Fisher Scientific, catalog number: 16140-089)
Nucleic acid extraction and preparation
Proteinase K (Biobasic, catalog number: PB0451)
Phase lock gel, heavy (VWR, catalog number: 10847-802)
DNase I (RNase-free) (NEB, catalog number: M0303L)
AmbionTM RNase III (Thermo Fisher Scientific, catalog number: AM2290)
RNase A (Qiagen, catalog number: 19101)
UltraPureTM DNase/RNase-free distilled water (Thermo Fisher Scientific, catalog number: 10977015)
Covaris microTUBE AFA fiber pre-split snap-cap 6*16 mm (Covaris, catalog number: 520045 )
Phenol-chloroform isoamyalcohol (Bioshop, catalog number: PHE512.400)
Chloroform (Bioshop, catalog number: CCL402.1)
Reagent alcohol (Sigma, catalog number: 277649-1)
Tris (Bioshop, catalog number: TRS001.10)
Acetic acid, glacial (Thermo Fisher Scientific, catalog number: 351271-212)
EDTA (Bioshop, catalog number: EDT002.500)
Sodium acetate (Bioshop, catalog number: SAA304.5)
Sodium chloride (Bioshop, catalog number: SOD002.10)
1× RNase H buffer (see Recipes)
TE (see Recipes)
DRIP
Anti-DNA–RNA Hybrid [S9.6] antibody (Kerafast, catalog number: ENH002, hybridomas are available through ATCC, HB-8730)
DynabeadsTM Protein G for Immunoprecipitation (Thermo Fisher Scientific, catalog number: 10004D)
NucleoSpin® Gel and PCR (Macherey-Nagel, catalog number: 740609.250)
DNA Clean & ConcentratorTM (Zymo Research, catalog number: D4014)
BSA, molecular biology grade (NEB, catalog number: B9000S)
10× DRIP binding buffer (see Recipes)
1× DRIP binding buffer (see Recipes)
DRIP elution buffer (see Recipes)
Library preparation and qPCR
NEBNext® Multiplex Oligos for Illumina® (Index Primers Set 1) (NEB, catalog number: E7735S)
NEBNext® UltraTM II Directional RNA Library Prep Kit for Illumina® (NEB, catalog number: E7760S)
RNase H (New England Biolabs (NEB), catalog number: M0297L)
PowerUp SYBR Green PCR master mix (Thermo Fisher Scientific, catalog number: A25741)
Agarose and SDS-PAGE gel preparation and staining
SYBRTM Gold Nucleic Acid Gel Stain (Thermo Fisher Scientific, catalog number: S11494)
SYPRO Ruby Solution (Lonza, catalog number: 50562)
Glycine (Bioshop, catalog number: GLN001.10)
0.5× TBE (see Recipes)
10× DNA loading buffer (see Recipes)
SDS-PAGE running buffer (see Recipes)
Protein loading buffer (see Recipes)
Plasmids
pHis-MBP-hRNaseH1 (Alecki et al., 2020)
pGEX6P1-hsRNASEH2BCA (Addgene, catalog number: 108693)
vg-pETBlue (Alecki et al., 2020)
Equipment
Typhoon Imager (GE Healthcare) for in-gel fluorescence imaging and phosphorimaging
Viaa7 PCR system (Thermo Fisher Scientific) for real-time PCR
Covaris E220 (Covaris) for nucleic acid sonication
Centrifuge Avanti® J-E (Beckman Coulter)
AKTA FPLC (or equivalent chromatography system)
HiLoad 20/60 Superdex 200 column (GE Healthcare, catalog number: GE28-9893-36) (available from Sigma)
Superdex 200 10/300 GL size exclusion column (GE Healthcare, catalog number: 28990944)
Sonicator (Sonics, Vibra cellsTM) (probe sonicator for cell lysis)
Nutator rotating platform to gently mix tubes
Bacterial shaker
10 cm Petri dish
Liquid nitrogen for flash freezing
-80°C Freezer
Nanodrop spectrophotometer
Water bath
24°C Incubator
27°C Shaking incubator for S2 cells; S2 cells can also be grown on plates at room temperature
Procedure
文章信息
版权信息
© 2021 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Alecki, C. and Francis, N. J. (2021). Identification of R-loop-forming Sequences in Drosophila melanogaster Embryos and Tissue Culture Cells Using DRIP-seq. Bio-protocol 11(9): e4011. DOI: 10.21769/BioProtoc.4011.
分类
分子生物学 > RNA
发育生物学 > 细胞生长和命运决定 > 分化
系统生物学 > 基因组学 > DRIP-seq
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link












