- EN - English
- CN - 中文
Spin Labeling of RNA Using “Click” Chemistry for Coarse-grained Structure Determination via Pulsed Electron-electron Double Resonance Spectroscopy
利用脉冲电子-电子双共振光谱测定粗粒结构的“点击”化学的RNA自旋标记
(*contributed equally to this work) 发布: 2021年05月05日第11卷第9期 DOI: 10.21769/BioProtoc.4004 浏览次数: 5979
评审: Angeliki GiannoulisYINNIAN FENGAnonymous reviewer(s)
Abstract
Understanding the function of oligonucleotides on a molecular level requires methods for studying their structure, conformational changes, and internal dynamics. Various biophysical methods exist to achieve this, including the whole toolbox of Electron Paramagnetic Resonance (EPR or ESR) spectroscopy. An EPR method widely used in this regard is Pulsed Electron-Electron Double Resonance (PELDOR or DEER), which provides distances in the nanometer range between electron spins in biomolecules with Angstrom precision, without restriction to the size of the biomolecule, and in solution. Since oligonucleotides inherently do not contain unpaired electrons, these have to be introduced in the form of so-called spin labels. Firstly, this protocol describes how nitroxide spin labels can be site-specifically attached to oligonucleotides using “Click” chemistry. The reaction provides little byproducts, high yields, and is conveniently performed in aqueous solution. Secondly, the protocol details how to run the PELDOR experiment, analyze the data, and derive a coarse-grained structure. Here, emphasis is placed on the pitfalls, requirements for a good dataset, and limits of interpretation; thus, the protocol gives the user a guideline for the whole experiment i.e., from spin labeling, via the PELDOR measurement and data analysis, to the final coarse-grained structure.
Graphical abstract:

Schematic overview of the workflow described in this protocol: First, the spin-labeling of RNA is described, which is performed as a "Click"-reaction between the alkyne-functionalized RNA strand and the azide group of the spin label. Next, step-by-step instructions are given for setting up PELDOR/DEER distance measurements on the labeled RNA, and for data analysis. Finally, guidelines are provided for building a structural model from the previously analyzed data.
Background
The function of biomolecules is rooted in their three-dimensional structure, dynamics, and interaction with other molecules. For example, proteins and oligonucleotides adopt structures that provide interaction sites or binding pockets for metal ions, organic ligands, and other oligonucleotides or proteins. Upon complex formation, the structure of the biomacromolecule may change, and it is this structural change that is the basis for function; thus, methods are needed that enable resolution of the structures along the trajectory of the conformational change. X-ray crystallography, nuclear magnetic resonance (NMR) spectroscopy, electron microscopy (EM), small-angle X-ray scattering (SAXS), atomic force microscopy (AFM), optical trap, and magnetic tweezers are powerful methods for obtaining the structures of biomolecules at atomic resolution (Salas et al., 2015; Lottspeich and Engels, 2018; Geffroy et al., 2018); yet, they also have their limitations. X-ray crystallography requires the biomolecule to be crystallized and is, as EM, limited in providing information on dynamics and conformational intermediates. NMR spectroscopy can follow the dynamics of biomolecules since it is performed in liquid solution, but is limited with respect to the size of the biomolecule. Complementary to these techniques, fluorescence microscopy (FM), Förster resonance energy transfer (FRET), and electron paramagnetic resonance (EPR) spectroscopy are biophysical methods that allow resolution of the dynamics of structural changes without size restriction and in solution (Yang et al., 2012; Lottspeich and Engels, 2018; Kuzhelev et al., 2018). In the toolbox of EPR spectroscopy (Schweiger et al., 2001; Goldfarb et al., 2018), a set of pulse sequences summarized under the term Pulsed Dipolar EPR Spectroscopy (PDS; Schiemann et al., 2007) measures the dipolar coupling between the spins of unpaired electrons. This coupling encodes the inter-spin distance, from which structural and dynamic information can be derived. Owing to the high sensitivity of PDS, measurements at biomolecular concentrations down to the nanomolar range are feasible (Fleck et al., 2020). Out of all PDS techniques, Pulsed Electron-Electron Double Resonance (PELDOR or DEER) is the most prominent and works in a distance range from 1.5 to 16 nm upon deuteration of the buffer and the whole protein (Schmidt et al., 2016) with Angstrom precision (Jeschke, 2012; Tsvetkov et al., 2019). PELDOR imposes no size restriction (Malygin et al., 2019), and can be performed on biomolecules in liquid (Yang et al., 2012) or frozen solution (Duss et al., 2014), in membranes (Dastvan et al., 2019), and within cells (Theillet et al., 2016). However, since PELDOR requires unpaired electrons and biomolecules are usually diamagnetic, techniques are needed for site-directed spin labeling (Shelke and Sigurdsson, 2014). Focusing on RNA oligonucleotides, there are two principal strategies for spin labeling (Ward and Schiemann, 2014): the first is the phosphoramidite approach, where a spin-labeled phosphoramidite is incorporated into the RNA strand during solid-phase synthesis (Beaucage et al., 1992; Lottspeich and Engels, 2018) and the second is the post-synthetic method, where a functionalized RNA strand is labeled after synthesis (Kerzhner et al., 2016). The major drawbacks of the phosphoramidite approach are the rather laborious synthesis of the spin-labeled phosphoramidite and the easy reduction of the label during RNA synthesis, leading to an EPR-silent state. For the post-synthetic strategy, RNA strands modified with a unique functional group can be obtained commercially and are then reacted with a spin label carrying the complementary functional group. However, labeling with high yields and efficient purification with minimal product loss can be challenging. Even though the length of commercially available RNA oligonucleotides is limited, there are well-established methods for obtaining longer RNA strands, e.g., via enzymatic ligation (Duss et al., 2014; Kerzhner et al., 2018).
In this protocol, built on the publications Kerzhner et al., 2018, Wuebben et al., 2019 and 2020, we first introduce a workflow for the post-synthetic spin labeling of RNA oligonucleotides based on the “Click” reaction. Here, the “Click” reaction is performed as a copper-(I)-catalyzed [2+3] cycloaddition between an alkyne-substituted 5’-uridine in the RNA strand and an azide group on a nitroxide label (Figure 1). The reaction is usually quantitative and fast, and the purification of the labeled RNA can be easily performed via reversed-phase High Performance Liquid Chromatography (HPLC). Secondly, the protocol outlines how to set up and perform the PELDOR measurement. Thirdly, it provides a workflow for data analysis and the transformation of the distances into structures. Importantly, possible pitfalls are highlighted and tips provided on how to overcome experimental difficulties, which are rarely found in the literature.
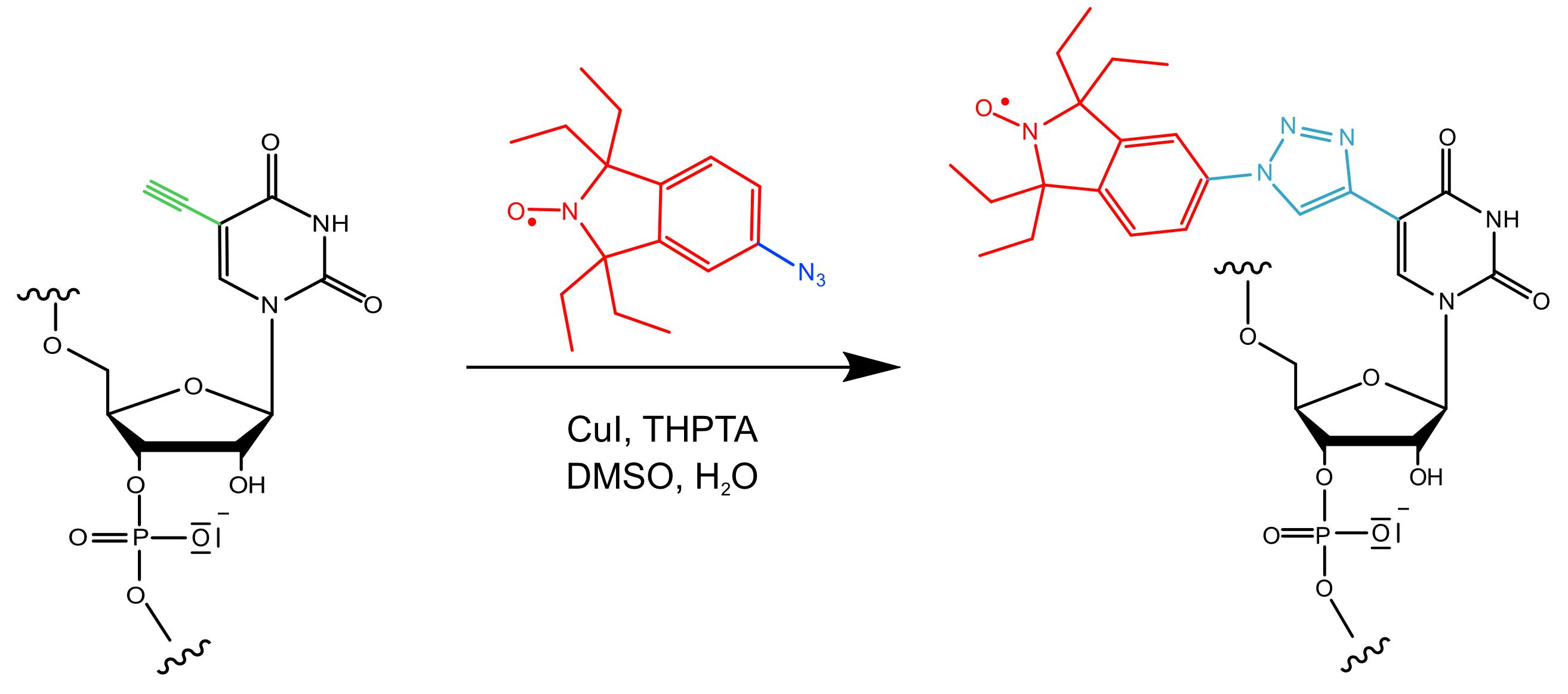
Figure 1. Reaction scheme of the “Click” reaction used in this protocol.The azide-functionalized spin label (azide group in blue; nitroxide moiety and 1,1,3,3-tetraethyl-5-azoisoindolin-2-oxyl backbone in red) is attached to a 5-ethynyl-2′-deoxyuridine nucleotide (ethynyl group in green) in the RNA strand (black). Copper, in its oxidation state (I), forms a complex with tris ((1-hydroxy-propyl-1H-1,2,3-triazol-4-yl) methyl) amine (THPTA), catalyzing this reaction in aqueous solution, which leads to the formation of a triazol linker (cyan) covalently connecting the nitroxide moiety to the RNA.
Beyond this application, labeling via “Click” chemistry is restricted neither to RNA nor to nitroxides or spin labels in general. It can be applied to e.g., DNA (El-Sagheer and Brown, 2010) and proteins (Nikić et al., 2015) and can be used for fluorescent labels (Liang et al., 2019). For protein labeling, one would make use of unnatural amino acids e.g., 4-ethynyl-L-phenylalanine (Widder et al., 2019). Note, however, that the reaction conditions will have to be adapted to the particular system under study. The workflow for the Q-band PELDOR experiment is universally applicable to measurements of nitroxide-labeled biomolecules. Likewise, the data analysis and distance-to-structure transformation can be adapted for other PDS techniques or other types of spin labels.
Materials and Reagents
Note: It is very important to set up an RNase-free environment. Therefore, use only autoclaved pipette tips, clean your bench and tools with 70% ethanol or RNase Away, and wear protective gloves.
RNase Away (Thermo Fisher, catalog number: 7003PK) or 70% Ethanol (Julius Hoesch GmbH, catalog number: 125900)
1.5 ml and 2 ml Eppendorf tubes
2.5 nmol dried RNA oligonucleotide with one or more 5-ethynyl-2’-deoxyuridine modifications (commercially synthesized and delivered in dried form e.g., from Metabion or Biomers, see procedure for more details), stored at -20°C (Note 1)
Diethylpyrocarbonate (DEPC, Carl Roth, catalog number: K028.3)-treated water
Copper(I)-iodide (Cu(I); Carl Roth, catalog number: 0305.1), stored at room temperature under ambient conditions in a regular container, as received
Dimethyl sulfoxide (DMSO, Carl Roth, catalog number: A994.2), stored at room temperature
3.6 µl 250 mM tris ((1-hydroxy-propyl-1H-1,2,3-triazol-4-yl) methyl) amine (THPTA, Sigma, catalog number: 762342-100MG) solution in DMSO, stored at -20°C, vortex the solution prior to use
2 µl 100 mM 1,1,3,3-tetraethyl-5-azoisoindolin-2-oxyl (Wuebben et al., 2019) or 1,1,3,3-tetramethyl-5-azoisoindolin-2-oxyl (Kerzhner et al., 2018) solution in DMSO, stored at -20°C, vortex the solution prior to use
Hamilton syringe, 50 µl
Buffer A, acetonitrile (Carl Roth, catalog number: 8825.2), stored at room temperature
Buffer B, 0.1 M triethylammonium acetate (TEAA) (1 M solution, Labomedic, catalog number: 2001741), stored at room temperature
For Liquid Chromatography-Mass Spectrometry (LC-MS): Buffer C, 10 mM triethylamine (Merck, catalog number: 90340-2.5L), stored at room temperature
For LC-MS, Buffer D, 0.1 M hexafluoroisopropanol (Merck, catalog number: 18127-50ML), stored at room temperature
For spin counting and determination of labeling efficiency: 10 µl capillaries (Hirschmann Laborgeräte, catalog number: L925.1)
Deuterated ethylene glycol (EG-d6) as a cryoprotectant (Merck, catalog number: 530549), stored at room temperature
Deuterated water (D2O) as a solvent (Deutero, order number: 00506), stored at room temperature
Pipette and matching elongated pipette tips (Sorenson BioScience, 200 µl MµltiFlex Round, catalog number: # 28480) to transfer the sample into the EPR tube
For PELDOR measurement: Q-band EPR sample tubes with 3 mm outer diameter made from clear-fused quartz (CFQ) (Wilmad LabGlass, catalog number: G542PN000001470)
Light-duty tissue wipers (VWR, European catalog number: 115-0202)
Liquid nitrogen (Air Liquide) for freezing the sample and liquid helium (Air Liquide) for cooling the sample in the resonator
DEPC-treated water (see Recipes)
Equipment
SpeedVac vacuum concentrator: Eppendorf Concentrator plus (Eppendorf, Rotor: F-45-48-11) or freeze dryer: Alpha 3-4 LSC basic (Martin Christ Gefriertrocknungsanlagen)
Thermomixer (Eppendorf)
Table-top centrifuge (Eppendorf)
Milli-Q Ultrapure Water System (Merck Millipore)
NanoDrop Spectrophotometer (Thermo Fisher)
High-Performance Liquid Chromatography (HPLC) system (Agilent, Series 1,200)
Reversed-phase C18 column, heatable (Zorbax 300SB-C18, 4.6 × 150 mm for RNA sequences shorter than 50 nucleotides or Zorbax 300SB-C18, 9.4 × 250 mm column for RNA sequences exceeding 50 nucleotides)
Amicon® Ultra-0.5 centrifugal filter device with a nominal molecular weight limit (NMWL) of 3,000 Da (Merck, catalog number: UFC500396)
Liquid Chromatography-Mass Spectrometry (LC-MS) system (HTC esquire from Bruker Daltonics in combination with an HPLC system). If you have no access to an LC-MS system, you can perform an analytical HPLC run and subsequently determine the exact mass via matrix-assisted laser desorption/ionization (MALDI) mass spectrometry or electrospray ionization (ESI) mass spectrometry
For spin counting and determination of labeling efficiency: continuous wave (cw) EPR spectrometer (Bruker BioSpin, model: EMXnano)
Pulsed EPR spectrometer (Bruker BioSpin, model: ELEXSYS E580) equipped with a Q-band microwave bridge (Bruker) and an ER5106-QT2 resonator (Bruker) for PELDOR measurements. When using an X-band resonator for continuous wave operation (e.g., Bruker ER4119HS), spin counting experiments can also be performed on this spectrometer
150 W travelling wave tube amplifier (TWT, Applied Systems Engineering, model: 187 Ka)
Liquid helium cryostat (Oxford Instruments, model: CF935)
Temperature controller (Oxford Instruments, model: iTC503S)
Helium transfer line (Oxford Instruments NanoScience, model: LLT600 or LLT650) and gas flow controller (Oxford Instruments)
Turbomolecular pump for evacuating the cryostat (Pfeiffer Vacuum, model: HiCUBE 80 Eco)
Membrane pump for maintaining a constant stream of cold helium gas (KNF Neuberger, model: PM26962–026.1.2)
Dewar vessels for shock-freezing and handling the EPR sample tubes at liquid nitrogen temperatures (KGW Isotherm, Type 00C, 13.5 cm in height; Type 3C, 26 cm in height)
100 L liquid helium tank (Cryo Anlagenbau, model CS 100 H)
Safety goggles and cold protection gloves
PC with Linux, as delivered with the spectrometer
Software
Matlab Version R2018a or later (The MathWorks Inc., www.mathworks.com). For DeerNet, the Deep Learning Toolbox and the Signal Processing Toolbox for Matlab should be installed
DeerAnalysis (e.g., DeerAnalysis 2019) toolbox for Matlab, available free of charge (G. Jeschke, https://epr.ethz.ch/software.html)
Software for data analysis and graphing, e.g., Origin (OriginLab Corporation, www.originlab.com), SciDAVIS (http://scidavis.sourceforge.net), or Excel (Microsoft Corporation, https://products.office.com/excel)
Xepr-software, as delivered with the EPR spectrometer, for hardware control and data acquisition (Bruker)
In silico spin labeling software, e.g., mtsslSuite (G. Hagelueken, www.mtsslsuite.isb.ukbonn.de; Hagelueken et al., 2015), MMM (G. Jeschke, https://epr.ethz.ch/software.html; Polyhach et al., 2011) or the GFN/FF-based CREST/MD (S. Grimme, https://github.com/grimme-lab; Spicher et al., 2020)
IDT OligoAnalyzer Tool (IDT, www.idtdna.com/pages/tools/oligoanalyzer)
SnrCalculator (D. Abdullin, https://github.com/dinarabdullin/SnrCalculator)
Procedure
文章信息
版权信息
© 2021 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Vicino, M. F., Hett, T. and Schiemann, O. (2021). Spin Labeling of RNA Using “Click” Chemistry for Coarse-grained Structure Determination via Pulsed Electron-electron Double Resonance Spectroscopy. Bio-protocol 11(9): e4004. DOI: 10.21769/BioProtoc.4004.
分类
生物物理学 > EPR波谱
生物化学 > RNA > RNA结构
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link