- EN - English
- CN - 中文
Screening for Lysogen Activity in Therapeutically Relevant Bacteriophages
筛选与治疗相关的噬菌体的溶原活性
发布: 2021年04月20日第11卷第8期 DOI: 10.21769/BioProtoc.3997 浏览次数: 9474
评审: Alba BlesaCristina SuárezNarayan Subramanian

相关实验方案
将Miniprep制备的大肠杆菌K12菌株质粒DNA转化为可用于植物遗传转化的根癌农杆菌EHA105细胞的简单可靠方法
Beenzu Siamalube [...] Steven Runo
2025年01月05日 2319 阅读
Abstract
Lysogenic phages can integrate into their bacterial host’s genome, potentially transferring any genetic information they possess including virulence or resistance genes, and are therefore routinely excluded from therapeutic applications. Lysogenic behavior is typically seen in phages that create turbid plaques or possess subpar bactericidal activity; yet, these are not definitive indicators. As a result, the presence of integrase genes is often used as a hallmark for lysogenic behavior; however, the accuracy of genetic screening for lysogeny depends on the quality of the extraction, sequencing and assembly of the phage genome, and database comparison. The present protocol describes a simple phenotypic test that can be used to screen therapeutically relevant phages for lysogenic behavior. This test relies on the identification of spontaneous phage release from their lysogenized host and can be reliably used in cases where no sequencing data are available. The protocol does not require specialized equipment, is not work-intensive, and is broadly applicable to any phage with an easily culturable bacterial host, making it particularly amenable to settings with limited resources.
Graphical abstract:
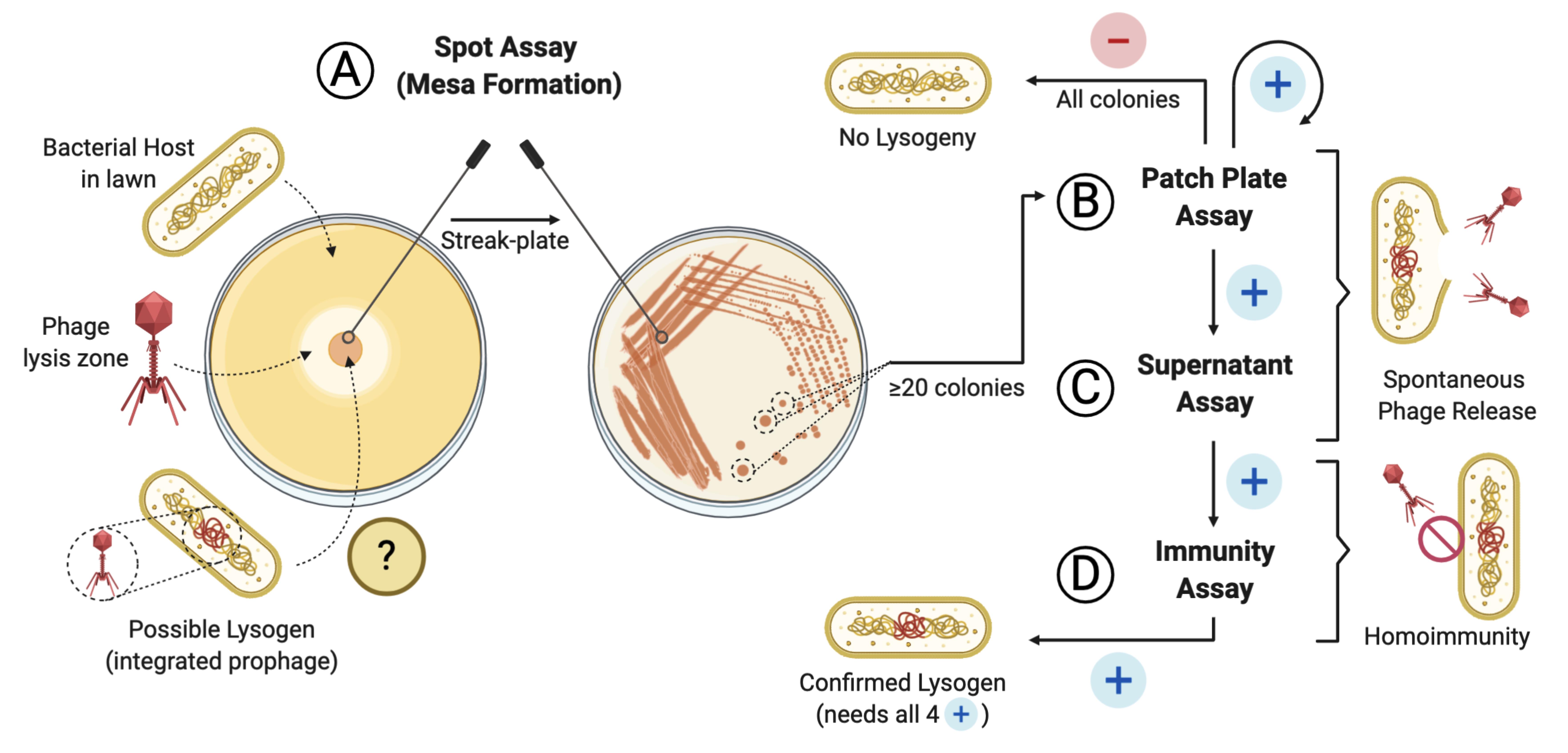
Screening pipeline for lysogen activity of a given phage
Background
The field of phage therapy, which is the clinical use of viruses to kill bacteria, has grown exponentially over recent decades (Gordillo Altamirano and Barr, 2019). Prior to their selection for therapeutic use, phages are typically subjected to a thorough characterization pipeline (Terwilliger et al., 2020). Lysogenic or temperate phages are those with the capacity to integrate themselves into their host’s genome, and are routinely excluded from therapeutic use due to their subpar bactericidal ability, the emergence of homoimmunity, and the potentially harmful consequences of lysogenic conversion (Fortier and Sekulovic, 2013; Hyman, 2019).
Temperate behavior is first suspected in phages that produce turbid plaques on bacterial lawns. However, plaque morphology can vary greatly depending on a variety of factors, including the growth medium used and the physiological state of the bacterial host (Ramesh et al., 2019). Delayed or substandard bactericidal activity in bacterial growth assays can also serve as an indicator, but their interpretation is not always clear-cut. Alternatively, genotypic screening for genes that are essential for lysogeny, such as integrase and excisionase enzymes, are further indicators of temperate behavior (Hyman, 2019). However, extraction and sequencing of phage genomes can be challenging, and the accuracy of homology-based analyses is highly dependent on the quality of the sequencing data, assemblies, and available databases (Russell, 2018). Importantly, the presence of a predicted integrase in the genome of a phage may not be definite confirmation of the ability to lysogenize a host in vitro or in vivo (Howard-Varona et al., 2017).
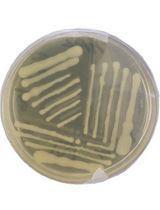
Here, we outline a simple protocol, modified from Pope et al. (2013), that evaluates the ability of a given phage to lysogenize its host in vitro. The assay can be reliably used in cases where no sequencing data are available. Furthermore, this protocol has the advantage of providing confirmation of lysogenic behavior for a given phage, instead of prediction, given by genome sequencing-based tests. It does not require specialized equipment and can complement analysis of plaque morphology and growth. Although we describe the protocol using an Acinetobacter baumannii-specific phage (Gordillo Altamirano et al., 2021), we also provide data from an Enterococcus faecium-specific phage (Figure 1), demonstrating that the methodology is broadly applicable to any phage with a bacterial host culturable on solid media. As such, incubation and media conditions may need be adapted to suit the host. Finally, in addition to excluding lysogenic phages from therapeutic use, the protocol can be used to produce lysogens (bacteria harboring a prophage) for downstream applications in phage biology and biotechnology research.
Materials and Reagents
15-ml polystyrene tubes (In Vitro Technologies, Falcon, catalog number: FAL352095)
Pipettes, serological pipettes, and pipette tips
10-ml sterile rimless glass test tubes (Thermo-Fisher, Youlyy, catalog number: LBSDCT13100)
Wire loop
10-ml syringes with detachable needle (McFarlane, Terumo, catalog number: 19046TE)
0.2-μm filters, attachable to syringes (Pall, catalog number: 4612)
Phage to be tested, high titer lysate (≥ 106 plaque-forming units [PFU]/ml)
Note: For a protocol on phage isolation and purification, see Bonilla et al., 2016.
1× phosphate-buffered saline (PBS) solution (Merck Australia, OmniPur, catalog number: US16506)
BactoTM Tryptone (Gibco, catalog number: 211705)
Granulated yeast extract (Merck Australia, Millipore, catalog number: 1037530500)
NaCl (Merck Australia, Supelco, catalog number: 1064040500)
Agar powder (Merck Australia, Millipore, catalog number: 05040)
Overnight culture in LB of the phage’s bacterial host (see Recipes for LB medium)
LB medium top agar (see Recipes)
LB medium agar plates (see Recipes)
Note: The growth media in 13-15 can be replaced to cater for the bacterial host’s growth requirements.
Equipment
Microwave (Panasonic, model: 32 L stainless steel, catalog number: NN-ST67JSQPQ)
37°C incubator
Shaking platform (Thermo Scientific, model: CO2-resistant shaker, catalog number: 88881102)
Bunsen burner
Benchtop centrifuge (Eppendorf South Pacific, model: 5810 R, catalog number: 5811000487)
Autoclave (Tuttnauer, model: vertical 110L, catalog number: 5050ELV-D)
Procedure
文章信息
版权信息
© 2021 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Gordillo Altamirano, F. L. and Barr, J. J. (2021). Screening for Lysogen Activity in Therapeutically Relevant Bacteriophages. Bio-protocol 11(8): e3997. DOI: 10.21769/BioProtoc.3997.
分类
微生物学 > 抗微生物试验 > 抗细菌试验
生物科学 > 生物技术 > 微生物技术
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link