- EN - English
- CN - 中文
Development of a Chemical Reproductive Aging Model in Female Rats
雌性大鼠化学生殖衰老模型的建立
发布: 2021年04月20日第11卷第8期 DOI: 10.21769/BioProtoc.3994 浏览次数: 7323
评审: Victor Rodrigues SantosGilliard LachAnonymous reviewer(s)
Abstract
Women are born with an abundant but finite pool of ovarian follicles, which naturally and progressively decreased during their reproductive years until menstrual periods stop permanently (menopause). Perimenopause represents the transition from reproductive to non-reproductive life. It is usually characterized by neuroendocrine, metabolic and behavioral changes, which result from a follicular depletion and reduced number of ovarian follicles. During this period, around 45-50 years old, women are more likely to express mood disorders, anxiety, irritability and vasomotor symptoms. The current animal models of reproductive aging do not successfully replicate human perimenopause and the gradual changes that occur in this phase. While the traditional rat model of menopause involves ovariectomy or surgical menopause consisting of the rapid and definitive removal of the ovaries resulting in a complete loss of all ovarian hormones, natural or transitional menopause is achieved by the selective loss of ovarian follicles (perimenopause period). However, the natural aging rodent (around 18-24 months) model fails to reach very low estrogen concentrations and overlaps the processes of somatic and reproductive aging. The chronic exposure of young rodents to 4-vinylcyclohexene diepoxide (VCD) is a well-established experimental model for perimenopause and menopause studies. VCD induces loss of ovarian small follicles (primary and primordial) in mice and rats by accelerating the natural process of atresia (apoptosis). The VCD, ovary-intact or accelerated ovarian failure (AOF) model is the experimental model that most closely matches natural human progression to menopause mimicking both hormonal and behavioral changes typically manifested by women in perimenopause.
Graphical abstract: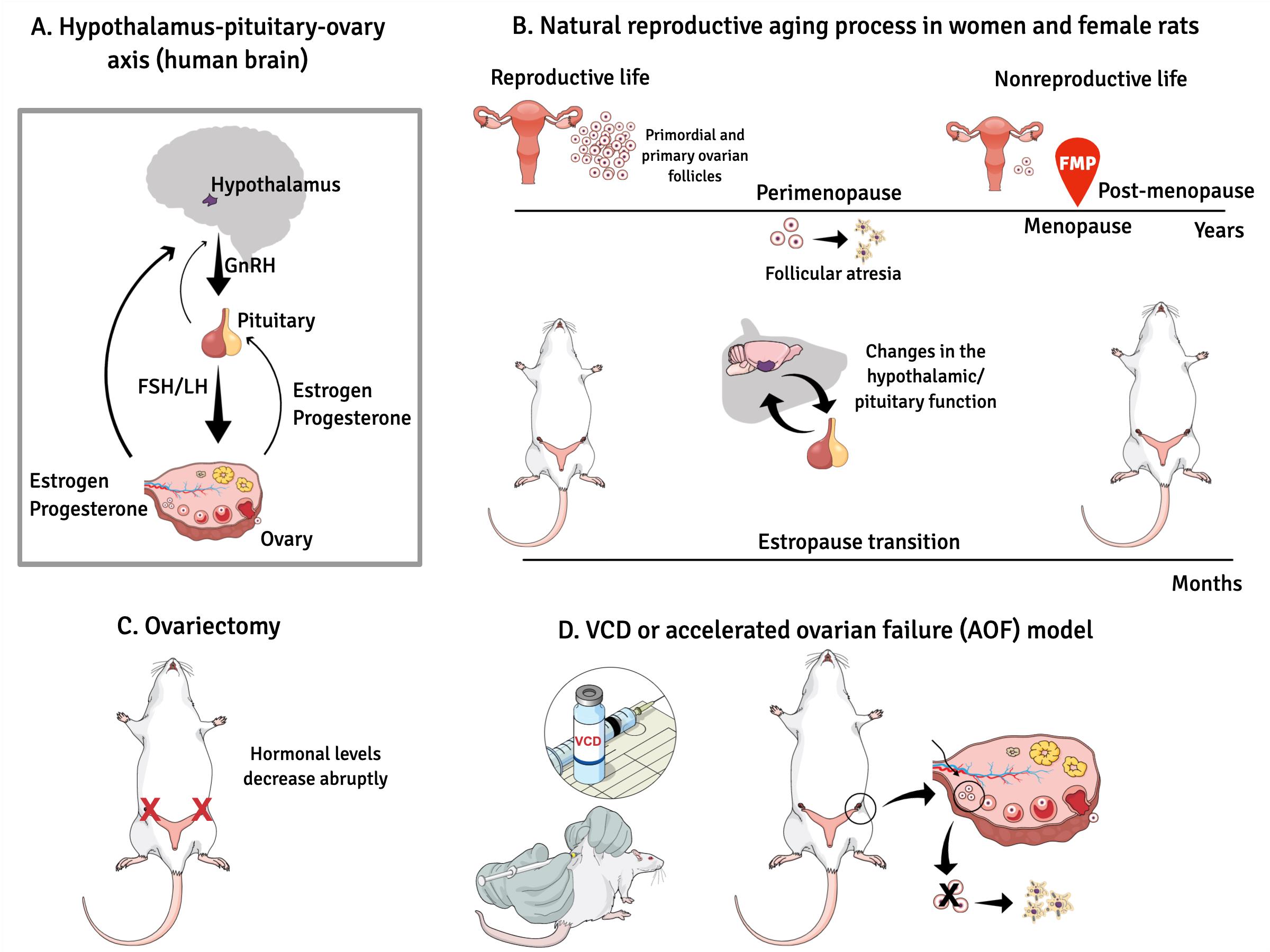
The female reproductive system is regulated by a series of neuroendocrine events controlled by central and peripheral components. (A). The mechanisms involved in this control are extremely complex and have not yet been fully clarified. In female mammals whose ovulation (the most important event in a reproductive cycle) occurs spontaneously, reproductive success is achieved through the precise functional and temporal integration of the hypothalamus-pituitary-ovary (HPO) axis. (B). In women, loss of fertility appears to be primarily associated with exhaustion of ovarian follicles, and this process occurs progressively until complete follicular exhaustion marked by the final menstrual period (FMP). (C). While in female rodents, reproductive aging seems to begin as a neuroendocrine process, in which changes in hypothalamic/pituitary function appear independently of follicular atresia. The traditional rat model of menopause, ovariectomy or surgical menopause consists of the rapid and definitive removal of the ovaries resulting in a complete loss of all ovarian hormones. (D). The chronic exposure (15-30 days) to the chemical compound 4-vinylcyclehexene diepoxide (VCD) in young rodents accelerates gradual failure of ovarian function by progressive depletion of primordial and primary follicles, but retains residual ovarian tissue before brain alterations that occurs in women in perimenopause. Low doses of VCD cause the selective destruction of the small preantral follicles of the ovary without affecting other peripheral tissues.
Background
Perimenopause, the transition period from reproductive to non-reproductive life, is defined as the period immediately before menopause. This period is marked by the onset of endocrine and biological changes, as well as clinical symptoms suggestive of the approach of menopause and can extend up to twelve months after the final menstruation, with an average duration of five years (WHO, 1996; Soules et al., 2001; Bacon, 2017; Wang et al., 2019). In addition to menopause or definitive cessation of menstrual cycling, perimenopause is a uniquely human process, but it can be mimicked by experimental models, especially in rodents. According to Prior and Hitchcock, perimenopause, previously seen as a period of hypoestrogenism, can be characterized by three main hormonal changes in women whose menstrual cycle remains regular: 1) normal or erratically high concentrations of estradiol; 2) decline in plasma progesterone concentrations and 3) changes at all levels of the reproductive axis (Prior and Hitchcock, 2011). During perimenopause a high percentage of women manifest typical symptoms of this period, which include: vasomotor changes, variations in menstrual cycle duration, sleep disorders, worsening cognitive functions, behavioral and mood changes (irritability, nervousness, anxiety and depression), in addition to metabolic and physiological changes (Mitchell and Woods, 1996; Brinton et al., 2015; Chalouhi, 2017). Considering the brain changes during this period, Brinton et al. (2015) defined perimenopause as a “state of neurological transition”.
The process of reproductive senescence in mammal species is complex and poorly understood, especially in humans (Brinton et al., 2009; Brinton, 2010). Consequently, animal models of menopause and perimenopause function as windows into the complex mechanisms involved in reproductive biology senescence at different levels (systemic, cellular, molecular, and genomic), which are not possible to perform in humans (Brinton, 2012). Nonetheless, current animal models of menopause do not successfully replicate human perimenopause and the gradual changes that occur in this phase. While the traditional rat model of menopause, ovariectomy or surgical menopause that consist of the rapid and definitive removal of the ovaries resulting in a complete loss of all ovarian hormones, natural or transitional menopause is achieved by the selective loss of ovarian follicle (perimenopause period). However, natural aging models fail to reach very low estrogen concentrations and overlapping changes related to somatic aging and those of reproductive aging (Kermath and Gore, 2012; Frye et al., 2012; Kirshner et al., 2020). Additionally, these models do not reproduce what occurs in women, since the primary causes of reproductive aging between species diverge significantly. In women, loss of fertility appears to be primarily associated with exhaustion of ovarian follicles (Faddy et al., 1992; Rubin, 2000), while in female rodents, reproductive aging seems to begin as a neuroendocrine process, with the changes in hypothalamic/pituitary function appearing independently of the follicular atresia (Gore et al., 2000).
A well-established experimental model in the literature for studying perimenopause and menopause is exposure of rodents to the chemical 4-vinylcyclohexene diepoxide (VCD), which leads a gradual failure of ovarian function by progressive depletion of primordial and primary follicles, but retains residual ovarian tissue similar to women in perimenopause (Springer et al., 1996; Kao et al., 1999; Hoyer et al., 2001). Importantly, the VCD model or model of Accelerated Ovarian Failure (AOF, Brooks et al., 2016) mimic both hormonal (Reis et al., 2014; Pestana-Oliveira et al., 2018; Carolino et al., 2019) and behavioral changes such as anxiety (Reis et al., 2014), impaired memory (Koebele et al., 2016), depression (Kalil et al., 2020) and aggressiveness (Dalpogeto et al., 2016; Scafuto et al., 2017) typically manifested by women in perimenopause. Low doses of VCD specifically cause selective destruction of ovarian small pre-antral follicles without affecting other peripheral tissues. Furthermore this occupational chemical doesn’t cross the blood-brain barrier (Lukefahr et al., 2012).
Therefore, the VCD-induced follicular depletion model, followed by ovarian failure has been widely used in experimental research on perimenopause and menopause (Reis et al., 2014; Liu et al., 2015; Brooks et al., 2016; Koebele et al., 2016; Pestana-Oliveira et al., 2018; Wang et al., 2019; Carolino et al., 2019; Kirshner et al., 2020) and is the experimental model that most closely matches the natural human progression to menopause, since the majority of women enter menopause through a gradual and irreversible process of reduction in ovarian function, while retaining the residual tissue of the ovary (Brooks et al., 2016). Thus, considering that it is a critical number of ovarian follicles and not the woman's age that determines the onset of menopause (Faddy et al., 1992), VCD-induced perimenopause is a translational model that presents analogy, predictability and homology, and allows plausible inferences to be made about the dynamics of follicular loss and its effects on the neurochemistry of women in perimenopause and menopause, periods in which affective disorders, vasomotor alterations and several other symptoms compromise the quality of life of middle-aged women.
Recently (2015-2017) the AOF model was applied to the streets and subways of large North American cities such as Chicago, New York, San Francisco, and Los Angeles with the aim of reducing the population of rats that has infested those cities (https://www.chicagomag.com/Chicago-Magazine/March-2015/birth-control-for-rats/).
Materials and Reagents
Polyethylene tubing (Thermo ScientificTM Immuno Tubes and Stoppers, catalog number: 12-565-150), stored at room temperature (RT)
Plastic funnel
Pipet tips (Eppendorf®, catalog numbers: 1300 RN [1-100 μl, yellow]; 1400 [101-1,000 μl, blue]), stored at room temperature
Glass slides for immunofluorescence (dimensions: size 25.4 x 76. 2 mm; thickness: 1.0 x 1.2 mm), twelve transparent circles (Perfecta, catalog number: 214-6), stored at RT
Flexible, translucent silicone elastomer tubing (Silastic, Dow CorningTM, 7.8 mm × 12.7 mm × 2.38, catalog number: 11-189-13B), stored at RT
Syringe (1 ml Tuberculin Syringe Regular Tip) (MonojectTM, catalog number: 8881501400), stored at RT
Sterile Gloves (Synthetical Surgical Gloves Powder-Free, Confiderm® SPT), stored at RT
Gauze sponges (Non-woven Gauze Sponges, 2 in. × 2 in. [5.08 cm × 5.08 cm]) (AVANT GAUZE®, catalog number: 25223), stored at RT
Needles (Standard Hypodermic Needles, MonojectTM, catalog numbers: 8881250255 [23G], 8881250149 [21G]), stored at RT
Females Wistar rats (age 28 days) from the animal facilities of the University of São Paulo, campus Ribeirão Preto, Brazil
4-vinylcyclohexene diepoxide (VCD [C8H12O2] Sigma-Aldrich, catalog number: 94956-250ML), stored at RT
17-β-estradiol (Sigma-Aldrich, catalog number: E8875-1G), stored at RT
Corn oil (Liza-900ML), stored at RT
Ketamine HCl Injection, USP (Ketaset®, 100 mg/kg; NDC: 0856-2013-1), RT
Xylazine (Schering-Plough, Coopers of Brazil, Cotia, São Paulo, 14 mg/kg), RT
Small Veterinary Pentabiotic® (Zoetis, 1.7 g/3 ml, catalog number: 232092),RT
Banamine Solution Injectable (Schering-Plough Animal Health, 2.5 mg/kg; catalog number: 12080097), stored at room temperature Sodium Cloride (NaCl) 0.9% (Samtec Biotecnology, 10 ml), RT
5% Povidone-iodine antiseptic microbicide for animal use (Betadine® Solution, catalog number: 12265), stored at RT
70% Isopropyl Alcohol (473 ml) (Medline Industries, catalog number: 53329-800-06), stored at RT
ELISA estradiol kit (DRG® Instruments GmbH DRG, EIA 2693), storage: 2 °C-8 °C
Progesterone double antibody RIA kit (P4) (MP Biomedicals, catalog number: SKU 07-170105 CF), storage: 2 °C-8 °C
Standard Rat Chow (23.2% Protein Rodent Diet, LabDiet®,catalog number: 5012), stored at RT
Heparin 50 units/ml (Heparin Sodium Injection USP, 50 units USP per ml) (B. Braun Medical Inc., catalog number: 0264-9577-10), stored at RT
4-vinylcyclohexene diepoxide dilution (VCD) (see Recipes)
Equipment
Standard cages (Bonther, 40 × 33 × 17 cm)
Guillotine (Bonther, model: Inox 420)
Surgical scissors
Forceps
Needle holder
Microscope (Zeiss Axioskop 2 Plus Ergonomic Trinocular, catalog number: 452342)
Centrifuge (Eppendorf, model: 5425, catalog number: 5405000042; 24 × 1.5/2.0 ml Capacity, up to 21, 330 × g (15,060 rpm), includes FA-24x2 rotor with aerosol-tight QuickLock lid, keypad control, 120 V)
Magnetic Stirrer Magnetic Stirrer with Heating (Thermo Scientific, catalog number: N2400-3010)
Shaker/Vortexer with racks (BenchMixer XLQ, catalog number: BV 1010-TST)
Detector Gamma Counter (Wizard2TM, PerkinElmer®, catalog number: 2470-0020)
Absorbance Reader 800 TS (BioTek)
Pipette single channel (EppendorfTM Research [0.5-10 µl, EP: 3123000020 yellow; 20-200 µl, EP: 312300055 yellow; 100-1,000 μl, EP: 312300063 blue]
Electronic Pipette Multichannel (EppendorfTM Research, 100 μl, SKU #184287407894)
Software
GraphPad Prism 7 software (GraphPad Software, La Jolla, CA)
Adobe Photoshop (Adobe Photoshop Lightroom, version 5.3; Adobe Systems, Inc.)
Mind the graph (www.mindthegraph.com)
Microsofit Excel
Procedure
文章信息
版权信息
© 2021 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Pestana-Oliveira, N., Carolino, R. O. G., Kalil, B., Mota Leite, C., Dalpogeto, L. C., De Paula, B. B., Collister, J. P. and Anselmo-Franci, J. A. (2021). Development of a Chemical Reproductive Aging Model in Female Rats. Bio-protocol 11(8): e3994. DOI: 10.21769/BioProtoc.3994.
- Pestana-Oliveira, N., Kalil, B., Leite, C. M., Carolino, R. O. G., Debarba, L. K., Elias, L. L. K., Antunes-Rodrigues, J. and Anselmo-Franci, J. A. (2018). Effects of Estrogen Therapy on the Serotonergic System in an Animal Model of Perimenopause Induced by 4-Vinylcyclohexen Diepoxide (VCD). eNeuro 5(1): ENEURO.0247-17.2017. doi: 10.1523/ENEURO.0247-17.2017.
分类
发育生物学 > 繁殖
神经科学 > 发育
生物科学 > 生物技术
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link










