- EN - English
- CN - 中文
Single-unit Recording in Awake Behaving Non-human Primates
非人类灵长类动物清醒时的单细胞记录
发布: 2021年04月20日第11卷第8期 DOI: 10.21769/BioProtoc.3987 浏览次数: 3045
评审: Atsushi NoritakeTomohiko TakeiAnonymous reviewer(s)
Abstract
Non-human primates (NHPs) have been widely used as a species model in studies to understand higher brain functions in health and disease. These studies employ specifically designed behavioral tasks in which animal behavior is well-controlled, and record neuronal activity at high spatial and temporal resolutions while animals are performing the tasks. Here, we present a detailed procedure to conduct single-unit recording, which fulfils high spatial and temporal resolutions while macaque monkeys (i.e., widely used NHPs) perform behavioral tasks in a well-controlled manner. This procedure was used in our previous study to investigate the dynamics of neuronal activity during economic decision-making by the monkeys. Monkeys’ behavior was quantitated by eye position tracking and button press/release detection. By inserting a microelectrode into the brain, with a grid system in reference to magnetic resonance imaging, we precisely recorded the brain regions. Our experimental system permits rigorous investigation of the link between neuronal activity and behavior.
Keywords: Non-human primates (非人灵长类动物)Background
Non-human primates (NHPs) are widely used as a species model in medical and life science research since they are the phylogenetically closest animal species to humans among those used for invasive experiments. In the field of systems neuroscience, research using NHPs have provided fundamental knowledge regarding neural mechanisms underlying the higher brain functions that are substantially evolved in humans, such as visual processing (Dubner and Zeki, 1971; Hubel and Wiesel, 1968; Perrett et al., 1982), decision making (Shadlen and Newsome, 2001; Padoa-Schioppa and Assad, 2006), working memory (Funahashi et al., 1989; Miller et al., 1996), attention (Treue and Maunsell, 1996; Luck et al., 1997), meta-cognition (Middlebrooks and Sommer, 2012; Miyamoto et al., 2017), and sociality (Gallese et al., 1996; Noritake et al., 2018). NHP research has also shown abnormal neural activity patterns in specific brain regions of animal models for neurological and psychiatric disorders, which are potentially physiological mechanisms underlying these disorders (Langston et al., 1984; Jentsch et al., 1997). These investigations provide insight into “where” and “how” the brain should be treated, and have therefore served as a basis for the development of therapeutic approaches for these disorders.
A remarkable achievement of neuroscience research using NHPs has been to advance our understanding of the relationship between neuronal activity and animal behavior. These studies employ specifically designed, well-controlled behavioral tasks, and record neuronal activity at high spatial and temporal resolutions while animals perform the tasks. These behavioral tasks need to finely control sensory inputs, accurately monitor behavioral responses, and precisely determine the time of task events. Neuronal activity recording has been implemented using several procedures. among which single-unit recording has been widely used due to its high spatial and temporal resolutions. By coupling these behavioral and recording procedures, NHP research has demonstrated that neuronal activities are tightly linked to specific sensory inputs, behavioral responses, and presumed internal processes at the single-unit level since the 1960s (Evarts, 1966; Wurtz, 1968).
Most NHP neuroscience research has been carried out in macaque monkeys, although other NHPs such as marmosets and squirrel monkeys have also been utilized. Our research group has conducted single-unit recording during which macaque monkeys performed behavioral tasks (Matsumoto and Takada, 2013; Kawai et al., 2015; Ogasawara et al., 2018). Figure 1 shows an overview of the experimental setup that allows collection of neuronal and behavioral data simultaneously across a unified timeline. Recently, we used this experimental setup to study the neural mechanisms underlying economic decision-making. We conducted single-unit recording of midbrain dopamine neurons and the orbitofrontal cortex while macaque monkeys performed an economic decision-making task (Figure 2A). In this task, six visual objects were associated with different amounts of a liquid reward, and two of them were randomly offered in sequence. When the first object was presented, the monkey needed to decide either to choose the first object by releasing the button or wait for the upcoming second object by continuing to press the button. The monkey obtained the reward associated with the chosen object at the end of the trial (Yun et al., 2020). This task design enabled us to continuously monitor neuronal activity while decisions were being made during the presentation of the first object, that is, as the monkey evaluated the first object, decided whether to choose it, and expressed the choice by releasing the button. We demonstrated that, while macaque monkeys were making an economic decision, midbrain dopamine neurons exhibited dynamically changing signals related to internal processing, such as value evaluation and identification of a chosen option (Yun et al., 2020). Here, we introduce the detailed experimental procedure used in this original study.

Figure 1. Schematic illustration of the entire behavioral and neuronal recording system.This illustration shows the configurations of apparatuses, how behavioral and neuronal data are collected by the TEMPO experimental control system, and how this system controls task events. The TEMPO experimental control system consists of three computers: Vsync, Server, and Client.
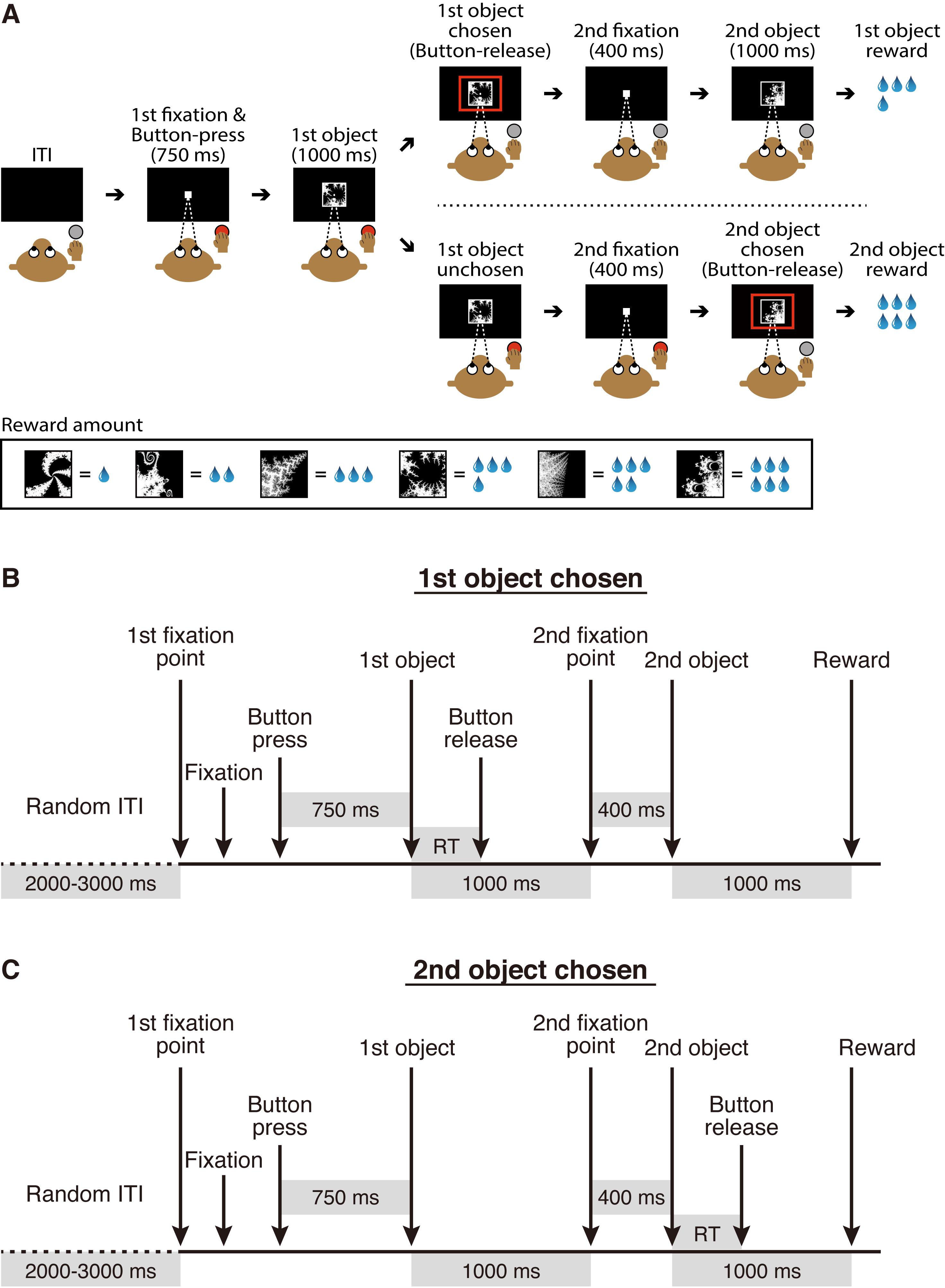
Figure 2. Economic decision-making task. A. Task design. This figure was reproduced with permission from our original paper (Yun et al., 2020. Figure 1A). B and C. The sequence of task events for trials in which a monkey decides to choose the first object or (B) the second object (C). ITI, intertrial interval; RT, choice reaction time.
Materials and Reagents
Materials
Recording chamber (Isekyu, custom-made, see Figure 3)
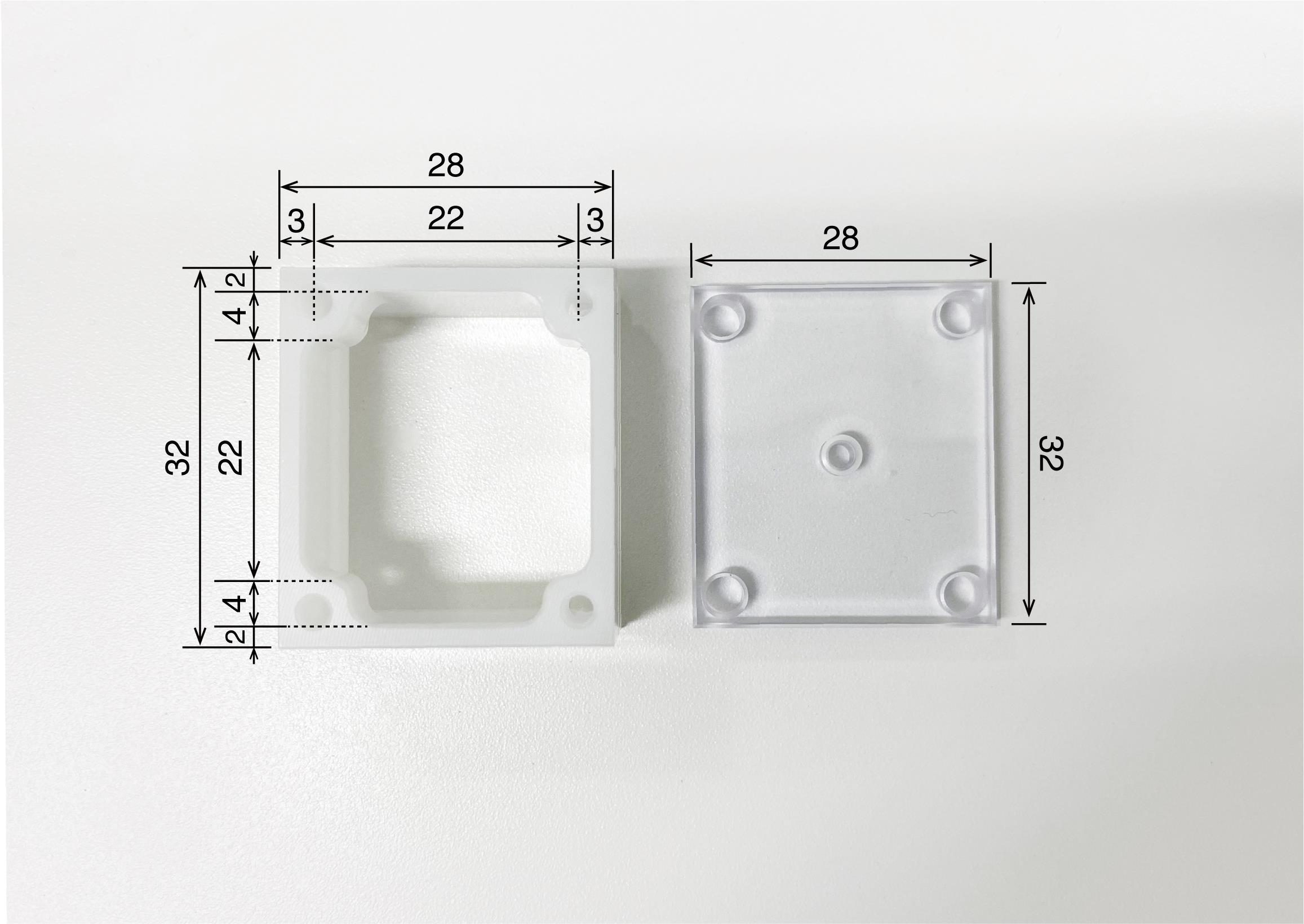
Figure 3. Recording chamber. Recording chamber (left) and its cover (right). The camber is made of polyoxymethylene and the cover is made of acrylic. The numbers written in the figure refer to millimeters as the unit.Recording grid (Isekyu, custom-made; see Figure 4)
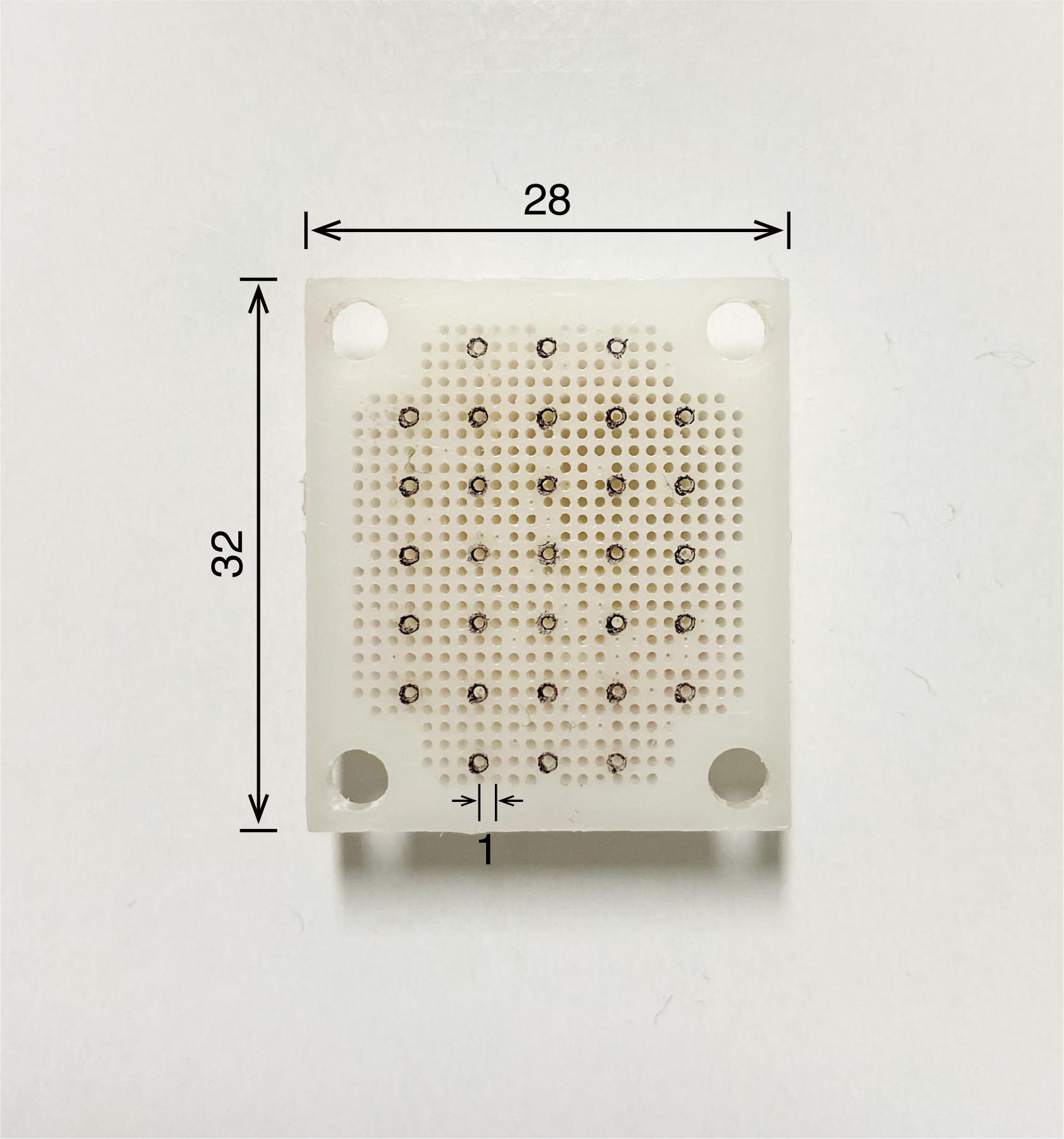
Figure 4. Recording grid. The grid is made of polyoxymethylene and needs to fit exactly in the recording chamber to accurately determine the position of microelectrode penetration. The numbers written in the figure refer to millimeters as the unit.Cortical screw (Wilco, catalog number: RY-0306; see Figure 5)
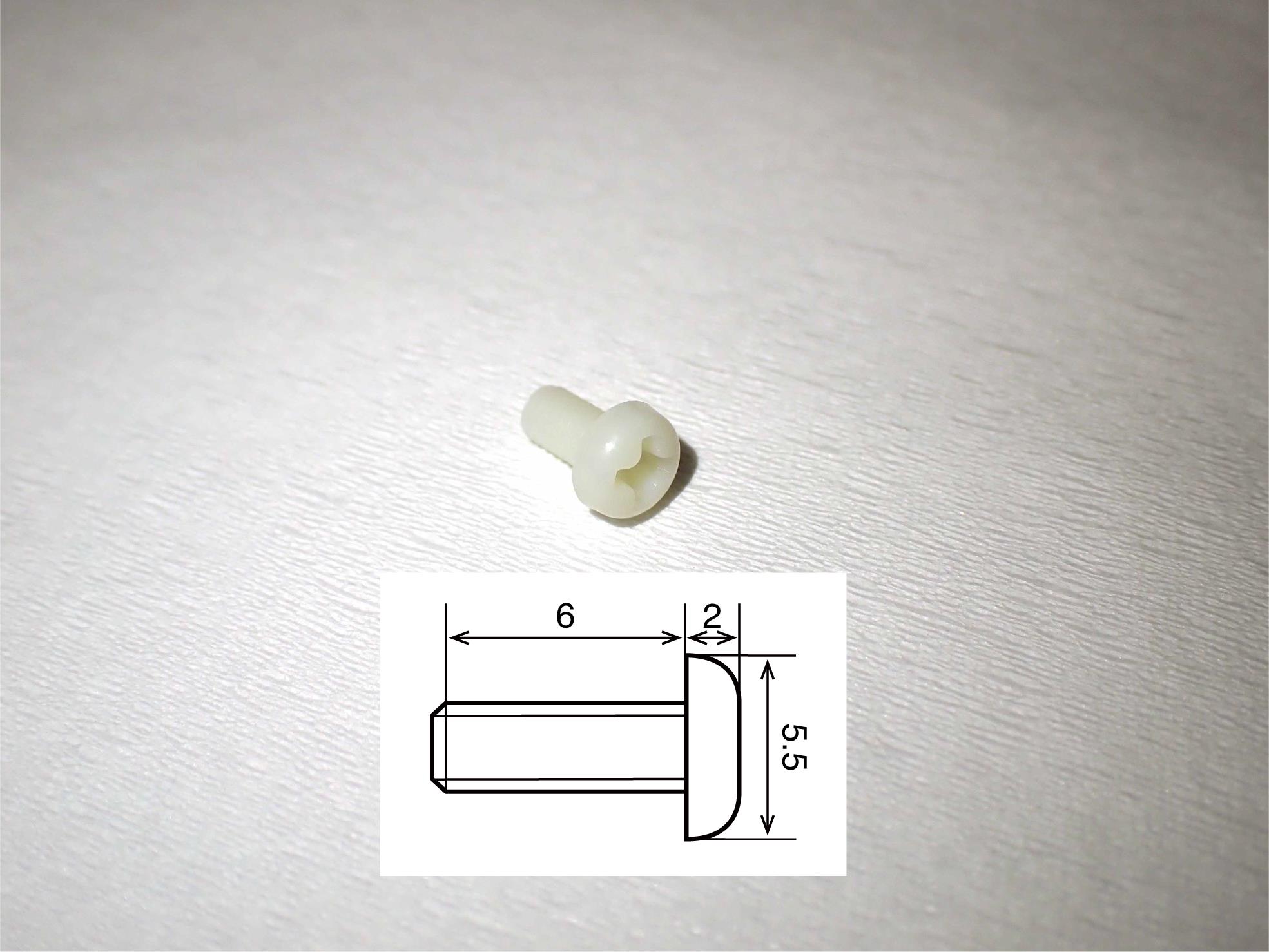
Figure 5. Cortical screw. The screw is made of polyamide MXD6, which is the main compound in reny. The schematic insert shows details of the size. The numbers written in the figure refer to millimeters as the unit.Head holder (Bioresearch Center, catalog number: CP1007-0031P-3, see Figure 6)

Figure 6. Head holder. Two of the cylinders are embedded in dental acrylic resin, which covers the top of the monkey’s skull. The two cylinders are configured in parallel. The cylinder is made of peek. The numbers written in the figure refer to millimeters as the unit.
Animals
Rhesus monkeys (Macaca mulatta)
Reagents
Saline (Otsuka Pharmaceutical, catalog number: 9F84S)
Chlorhexidine (Rapotec) (Nikko Pharmaceutical, catalog number: 872619)
Ethanol (FUJIFILM Wako Chemical, catalog number: ESL2739)
Povidone iodine (Popyral) (Nikko Pharmaceutical, catalog number: 2612701Q3407)
Ketamin (Ketalar) (Daiichi Sankyo Propharma, catalog number: 1119400A2038)
Pentobarbital sodium (Somnopentyl) (Kyoritsu Seiyaku, catalog number: SOM03-ON1706)
Xylazine (Seractal) (Bayer Medical, catalog number: 4987341106720)
Cefazolin sodium (Sefmazon) (Nipro Pharmaceutical, catalog number: 876132)
Buprenorphine hydrochloride (Lepetan) (Otsuka Pharmaceutical, catalog number: 31641-1)
Atropine sulfate hydrate (Nipro Pharmaceutical, catalog number: 1242405A1011)
Dental acrylic resin (Repairsin) (GC, catalog number: 20300BZZ00570000)
Ointment (CHLOMY-P) (Alfresa Pharmaceutical, catalog number: EAR2224)
Equipment
Battery-operated impedance meter (World Precision Instruments, model: OMEGAZ)
Binocular biological microscope (Beijing Tech Instrument, catalog number: 0702-327)
Tungsten microelectrode (FHC, catalog number: UEWLEESEEN1E)
Stainless-steel guide tube (original design, see Figure 7)
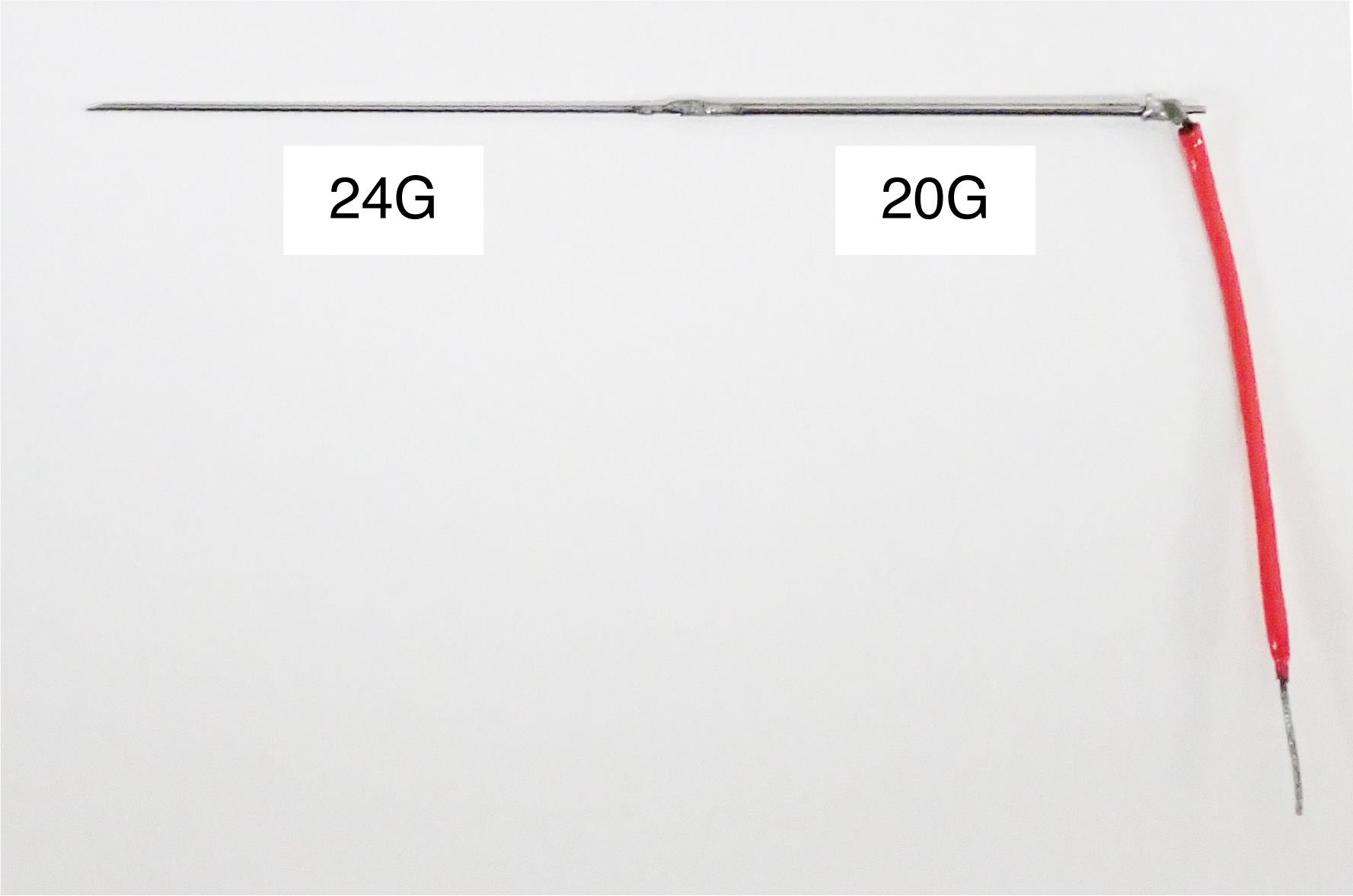
Figure 7. Stainless-steel guide tube. The guide tube is used to guide a microelectrode into the brain via the dura. It is made of stainless-steel tubes of two gage sizes (20G and 24G). The 24G part is inserted into the brain and the 20G part is firmly attached to an oil-driven micromanipulator (see Figure 12). The length of each part is adjusted according to the depth of the recording site. The red wire is connected to the minus pole of the pre-amplifier of the multi-channel processor.Uninterruptible power supply (APC, catalog number: 0L1039)
Oscilloscope (HITACHI, catalog number: V-250)
Multi-channel processor (Alpha Omega, model: MCP-Plus 8)
Voltage–time window discrimination system (Alpha Omega, model: ASD)
Reward system (Crist Instrument, model: 5-RLD-E2)
50/60 Hz noise eliminator (Quest Scientific Instruments, model: HumBug HB5060HZ)
Infrared eye tracking system (SR Research, model: EyeLink1000 Primate)
Computer monitor (Dell, model: P2412Hb)
Oil-driven micromanipulator (Narishige Scientific Instrument Lab, catalog number: MO-97-S)
Hand-driven micromanipulator (KOPF Instruments, catalog number: 7456B)
Monkey chair with a button (O’hara, catalog number: MC-3404MS; see Figure 8)
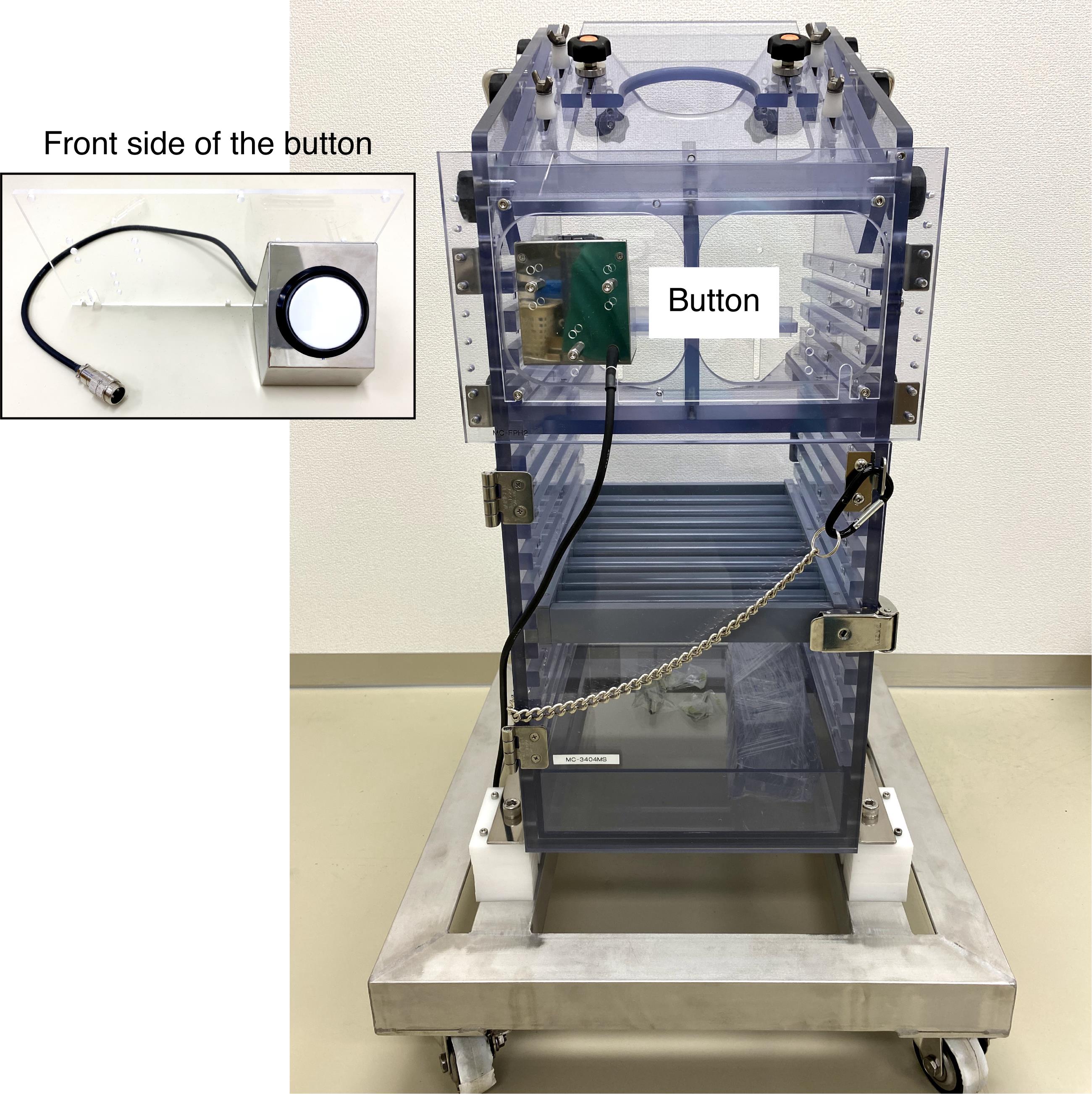
Figure 8. Monkey chair with a button. The image insert shows the front side of the button.Sound-attenuated and electrically shielded room (O’hara, custom-made)
Steel frame (O’hara, custom-made, modelr: MC-BFCS; see Figure 9)
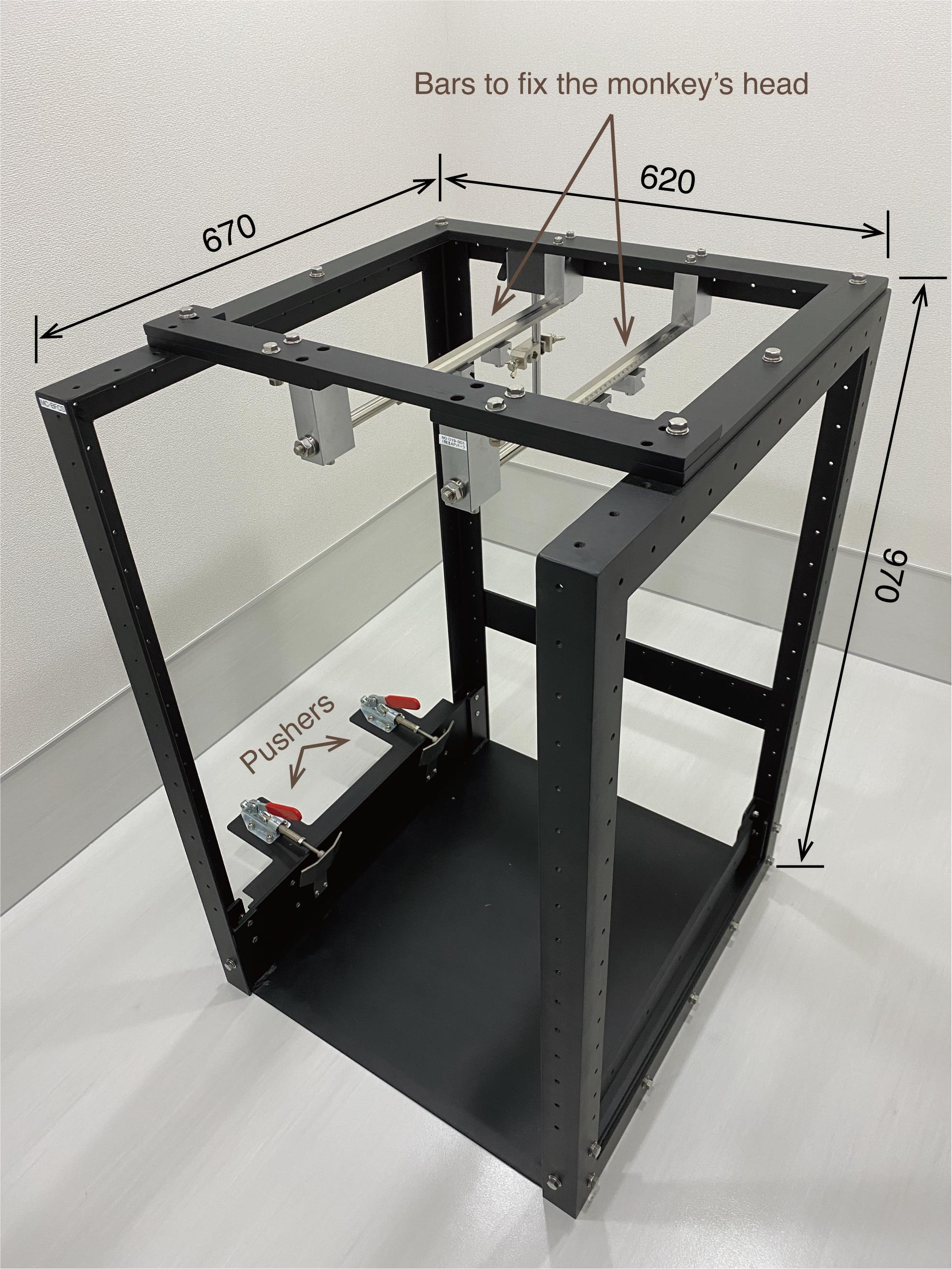
Figure 9. Steel frame. The monkey chair is firmly anchored by the two pushers. The monkey’s head is fixed to the two bars attached to the top of the steel frame. The computer monitor is placed in front of the steel frame (not shown here). The numbers written in the figure refer to millimeters as the unit.
Software
Multi-channel processor (MCP) ver 3.73 (ALPHA OMEGA, www.alphaomega-eng.com)
Alpha spike detector (ASD) (ALPHA OMEGA, www.alphaomega-eng.com)
TEMPO experimental control system (Reflective Computing, http://reflectivecomputing.com)
EyeLink (SR Research, http://sr-research.jp)
Matlab (Mathworks, http://mathworks.com)
Procedure
文章信息
版权信息
© 2021 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Yun, M., Nejime, M. and Matsumoto, M. (2021). Single-unit Recording in Awake Behaving Non-human Primates. Bio-protocol 11(8): e3987. DOI: 10.21769/BioProtoc.3987.
- Yun, M., Kawai, T., Nejime, M., Yamada, H. and Matsumoto, M. (2020). Signal dynamics of midbrain dopamine neurons during economic decision-making in monkeys. Sci Adv 6(27): eaba4962.
分类
神经科学 > 基础技术
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
提问指南
+ 问题描述
写下详细的问题描述,包括所有有助于他人回答您问题的信息(例如实验过程、条件和相关图像等)。
Share
Bluesky
X
Copy link