- EN - English
- CN - 中文
Production of Phenotypically Uniform Human Cerebral Organoids from Pluripotent Stem Cells
从多能干细胞生产表型一致的人类大脑类器官
(*contributed equally to this work) 发布: 2021年04月20日第11卷第8期 DOI: 10.21769/BioProtoc.3985 浏览次数: 5543
评审: Prashanth N SuravajhalaObul BandapalliAnonymous reviewer(s)
Abstract
Recent advances in stem cell technology have allowed researchers to generate 3D cerebral organoids (COs) from human pluripotent stem cells (hPSCs). Indeed, COs have provided an unprecedented opportunity to model the developing human brain in a 3D context, and in turn, are suitable for addressing complex neurological questions by leveraging advancements in genetic engineering, high resolution microscopy, and tissue transcriptomics. However, the use of this model is limited by substantial variations in the overall morphology and cellular composition of organoids derived from the same pluripotent cell line. To address these limitations, we established a robust, high-efficiency protocol for the production of consistent COs by optimizing the initial phase of embryoid body (EB) formation and neural induction. Using this protocol, COs can be reproducibly generated with a uniform size, shape, and cellular composition across multiple batches. Furthermore, organoids that developed over extended periods of time (3–6 months) showed the establishment of relatively mature features, including electrophysiologically active neurons, and the emergence of oligodendrocyte progenitors. Thus, this platform provides a robust experimental model that can be used to study human brain development and associated disorders.
Graphic abstract:
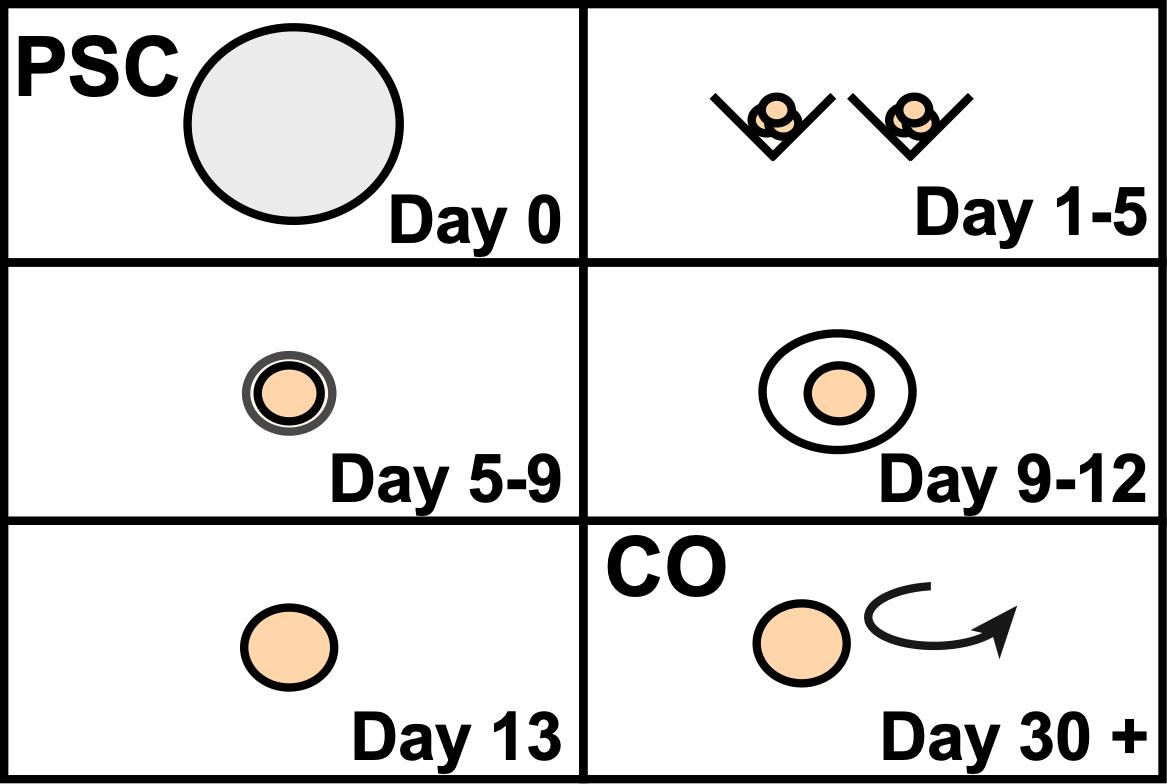
Overview of cerebral organoid development from pluripotent stem cells
Background
Recent advancements in the in vitro development of 3D cerebral organoids (COs) derived from human pluripotent stem cells (hPSCs) have provided an unprecedented opportunity to model the developing human brain and relevant complex diseases in an experimentally tractable system. Indeed, this approach has allowed researchers to study early brain development and the consequences of alterations associated with various human neurological disorders, such as Alzheimer’s, blindness, Autism Spectrum Disorder (ASD), and Zika virus infection (Lancaster and Knoblich, 2014b; Quadrato et al., 2016; Di and Kriegstein, 2017; Huch et al., 2017; Amin and Paşca, 2018; Rossi et al., 2018; Chen et al., 2019). In addition, several groups have applied COs to study and establish preclinical models of human brain cancers such as glioblastoma multiforme (Drost and Clevers, 2018; Linkous et al., 2018). In recent years, numerous protocols have emerged to facilitate the development of region specific-COs by controlling the underlying cell signaling pathways with exogenous growth factors and small molecule inhibitors to guide cell fate changes as the organoid matures (Lancaster et al., 2013; Mariani et al., 2015; Jo et al., 2016; Qian et al., 2016; Birey et al., 2017; Quadrato et al., 2017; Watanabe et al., 2017; Pollen et al., 2019; Velasco et al., 2019; Yoon et al., 2019). However, due to the fact that human whole-brain organoids are largely produced by intrinsic self-patterning and do not rely on controllable exogenous factors, stochastic differentiation often leads to cellular diversity, which is amplified with extended culture. Unfortunately, the considerable variability between individual organoids obtained using whole-brain differentiation platforms can therefore limit the utility of these COs for studying disease mechanisms or the development of potential therapeutics. Here, we describe our robust protocol for efficiently and reproducibly generating mature, uniform human COs (Figure 1). By optimizing an established protocol for the creation of self-patterned whole-brain organoids (Lancaster and Knoblich, 2014a; Lancaster et al., 2013), we successfully generated phenotypically uniform forebrain organoids with reproducible cell-type compositions.
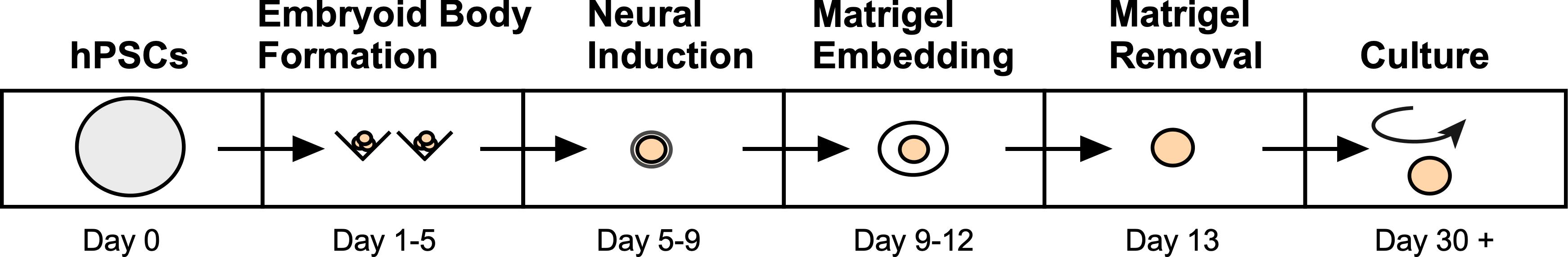
Figure 1. Overview of the developed method to generate human COs from pluripotent stem cells
Materials and Reagents
96-well V-bottomed non-binding plates (Greiner Bio-One, catalog number: 651970 )
24-well clear flat-bottomed ultra-low attachment plates (Corning, catalog number: 3473 )
4-well cell culture plates (ASI, catalog number: TP9004 )
50 ml and 15 ml conical centrifuge tubes (Corning, Falcon, catalog numbers: 352096, 352070 )
Low-retention microcentrifuge tubes (1.5 ml and 0.6 ml, Thermo Fisher Scientific, catalog numbers: 3451 and 3446 )
50 ml sterile disposable reagent reservoirs (Corning, catalog number: 4870 )
DMEM/F-12, HEPES (Thermo Fisher Scientific, Gibco, catalog number: 11330032 , store at 4 °C)
KnockOutTM Serum Replacement – Multi-Species (Thermo Fisher Scientific, Gibco, catalog number: A3181502 , store at -20°C)
MEM Non-Essential Amino Acid Solution (100×) (Thermo Fisher Scientific, Gibco, catalog number: 11140050 , store at 4°C)
2-Mercaptoethanol (1,000×) (Thermo Fisher Scientific, Gibco, catalog number: 21985023 , store at 4 °C)
Animal-Free Recombinant Human FGF-basic (Peprotech, catalog number: AF-100-18B , store at -80°C)
TrypLETM Express Enzyme (1×) (Thermo Fisher Scientific, Gibco, catalog number: 12604013 , stored at room temperature [22°C] in the dark)
Neural Basal Medium (Thermo Fisher Scientific, Gibco, catalog number: 21103049 , store at 4°C)
Y27632 ROCK Inhibitor (Cedarlane, catalog number: S1049-10MG , store at -80°C)
GlutaMAXTM Supplement (Thermo Fisher Scientific, Gibco, catalog number: 35050-061 , store at 4°C)
Heparin sodium salt (Sigma-Aldrich, catalog number: H4784 , store at -20°C)
Insulin solution human (Sigma-Aldrich, catalog number: I9278 , store at 4°C)
N-2 Supplement (100×) (Thermo Fisher Scientific, Gibco, catalog number: 17502001 , aliquots of 500 µl, store at -20°C)
B-27TM Supplement (50×), minus vitamin A (Thermo Fisher Scientific, Gibco, catalog number: 12587010 , aliquots of 500 µl, store at -20°C)
B-27TM Supplement (50×), serum-free (Thermo Fisher Scientific, Gibco, catalog number: 17504044 , aliquots of 500 µl, store at -20°C)
Matrigel® Growth Factor Reduced (GFR) Basement Membrane Matrix (Corning, catalog number: 356231 , aliquots of 500 µl, store at -20°C)
D-PBS-/-, 1×, without calcium and magnesium (Wisent Bioproducts, catalog number: 311-425-CL , store at 4°C)
Embryoid Body Media (EB Media) (see Recipes)
Neural Induction Media (see Recipes)
Cerebral Organoid Differentiation Media without Vitamin A (CDM-A) (see Recipes)
Cerebral Organoid Differentiation Media with Vitamin A (CDM+A) (see Recipes)
Equipment
NalgeneTM Square PETG media bottles (250 ml) (Gibco, Thermo Fisher Scientific, catalog number: 2019-0250 )
Pipettes (5 ml, 10 ml, 25 ml, 50 ml), micro-pipettes (10 µl, 20 µl, 200 µl, 1,000 µl)
Multi-channel pipette (Eppendorf, model: Research plus 12 channel pipette, catalog number: ES-12-300 )
Water bath
Centrifuge
Phase contrast microscope
Hemocytometer
37°C, 5% CO2 cell culture incubator
Orbital shaker that can be installed inside the incubator (such as Thermo Fisher, catalog number: 88881101 )
Blade and scalpel
Procedure
文章信息
版权信息
© 2021 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Sivitilli, A., Ghiasi, P. and Attisano, L. (2021). Production of Phenotypically Uniform Human Cerebral Organoids from Pluripotent Stem Cells. Bio-protocol 11(8): e3985. DOI: 10.21769/BioProtoc.3985.
分类
发育生物学 > 形态建成
神经科学 > 基础技术
细胞生物学 > 细胞工程 > 组织工程
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link