- EN - English
- CN - 中文
Ex vivo Assessment of Mitochondrial Function in Human Peripheral Blood Mononuclear Cells Using XF Analyzer
使用XF分析仪体外评估人外周血单个核细胞线粒体功能
发布: 2021年04月05日第11卷第7期 DOI: 10.21769/BioProtoc.3980 浏览次数: 5178
评审: Luis Alberto Sánchez VargasAlexandros C KokotosAnonymous reviewer(s)

相关实验方案

基于 T 细胞的平台,用于对肿瘤浸润 T 细胞的单细胞 RNA 测序数据集中鉴定的 T 细胞受体进行功能筛选
Aaron Rodriguez Ehrenfried [...] Rienk Offringa
2024年04月20日 6409 阅读

研究免疫调控血管功能的新实验方法:小鼠主动脉与T淋巴细胞或巨噬细胞的共培养
Taylor C. Kress [...] Eric J. Belin de Chantemèle
2025年09月05日 3550 阅读
Abstract
Cellular health and function, as we know today, depend on a large extent on mitochondrial function. The essential function of mitochondria is the energy production, more precisely ATP production, via oxidative phosphorylation. Mitochondrial energy production parameters therefore represent important biomarkers. Studies on human cells have mainly been performed on in vitro cell cultures. However, peripheral blood mononuclear cells (PBMCs) are particularly suitable for such examinations. That’s why this protocol describes a method to measure key parameters of mitochondrial function in freshly isolated PBMCs with the latest technology, the XF Analyzer. For this ex vivo approach PBMCs are first isolated out of human anticoagulated blood. Next, they are attached to the surface of special microplates pre-coated with Poly-D-Lysine. During the subsequent measurement of oxygen consumption rate (OCR) as well as extracellular acidification rate (ECAR) the stress reagents oligomycin, carbonyl cyanide 4-(trifluoromethoxy)phenylhydrazone (FCCP), rotenone and antimycin A are injected. Several mitochondrial parameters can be calculated from the results obtained. The application of this protocol allows the analysis of various influences, such as pharmaceuticals or environmental factors, on human cells.
Keywords: Human peripheral blood mononuclear cells (PBMCs) (人外周血单核细胞)Background
Mitochondria play a critical role in maintaining normal cellular function. It is now common knowledge that they not only produce ATP via oxidative phosphorylation but, for example, are also involved in the metabolism of amino acids, lipids and nucleotides, diverse signaling and redox processes as well as quality control and degradation processes including mitophagy and apoptosis (Pfanner et al., 2019). However, mitochondria represent the major site of ATP synthesis in normal cells (Akbari et al., 2019). For this purpose, an electrochemical proton gradient is generated across the mitochondrial inner membrane through the multi-subunit enzyme complexes I–IV. This proton gradient is used by the ATP synthase, also known as complex V, to turn ADP into ATP (Chaban et al., 2014).
The process of oxidative phosphorylation is associated with the reduction of oxygen to water. Accordingly, the oxygen consumption rate of cells can be used for assessing mitochondrial function (Smolina et al., 2017). This principle is the basis of Seahorse XF Analyzers (Agilent Technologies). They provide the possibility to measure not only oxygen consumption rate (OCR), but also the rate of extracellular acidification (ECAR), which is a key indicator of glycolysis. The realtime measurements are carried out in multi-well plates which are provided with solid-state sensors consisting of two fluorophores. One is quenched by oxygen (O2) and the other one is sensitive to pH-value changes. The fluorophores are excited via light-emitting fiber optic bundles, which subsequently detect the fluorescence changes as a result of oxygen consumption or extracellular acidification (Plitzko and Loesgen, 2018). Furthermore, XF Analyzers enable up to four different injections per well during the measurement. All the properties mentioned constitute a significant advantage over the conventionally used clark-type oxygen electrodes for determining oxygen consumption.
Peripheral blood mononuclear cells (PBMCs) as sample in a XF Analyzer implies that the cells have to be attached to the surface of the microplates. Most commonly, this immobilization is done by means of protein solutions such as Cell-TakTM (Jones et al., 2015; Traba et al., 2016; Lee et al., 2019) or Poly-D-Lysine (Hartman et al., 2014; Nicholas et al., 2017; Thaventhiran et al., 2019). We examined both Cell-TakTM and Poly-D-Lysine and considered Poly-D-Lysine as most suitable coating method. Since we conducted the Agilent Seahorse XF Cell Mito Stress Test, optimal concentrations of the injected compounds oligomycin, FCCP, antimycin A and rotenone had to be tested as well. A typical curve of a Mito Stress Test is shown in Figure 1. Since oligomycin is an inhibitor of ATP synthase, OCR decreases after its injection. In contrast, OCR increases sharply after FCCP injection, which is an uncoupler of oxidative phosphorylation. The last injection of antimycin A and rotenone, again leads to a decline of OCR, as these two compounds inhibit complex III respectively I of the electron transport chain. The resulting curve is used to calculate various parameters of mitochondrial function (see Figure 1).
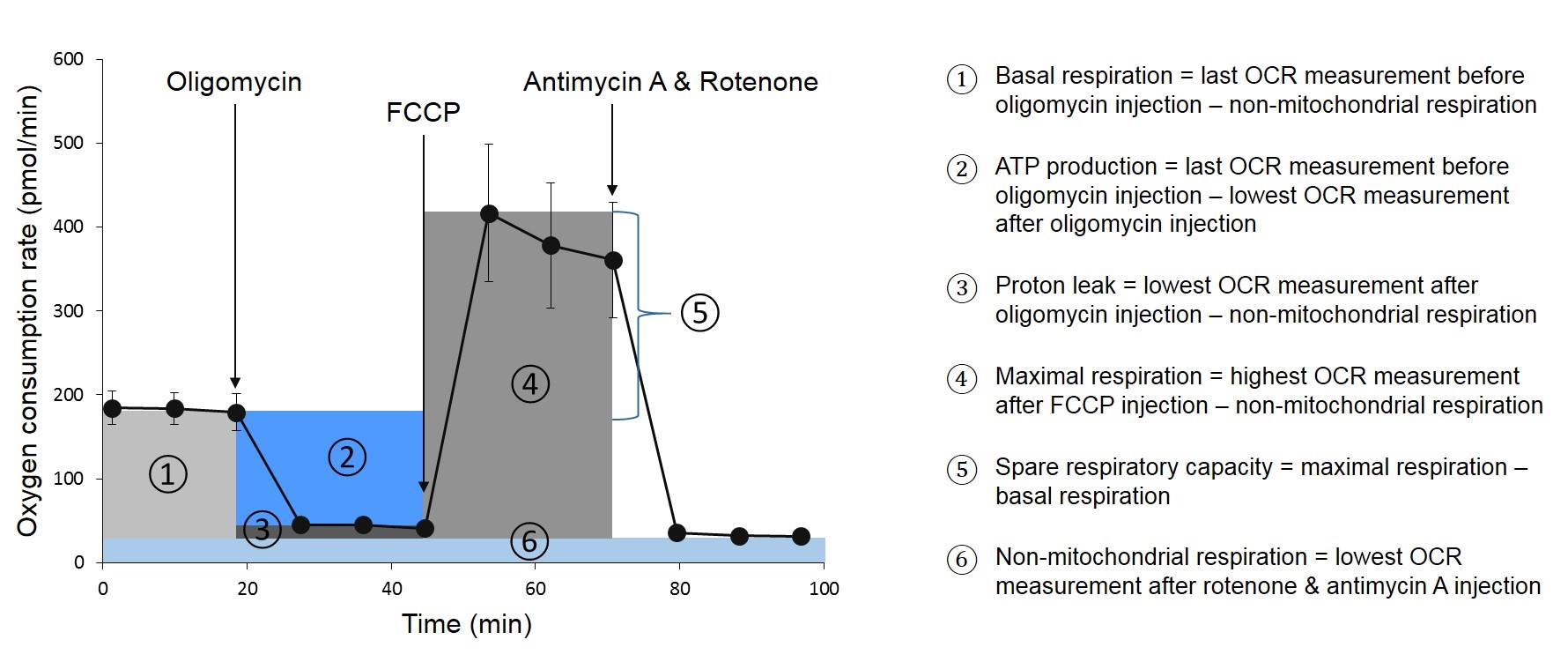
Figure 1. Assessment of mitochondrial respiration parameters by means of Agilent Seahorse XF Cell Mito Stress Test. On the left side a typical course of an OCR measurement with injections of oligomycin after the third, FCCP after the sixth and antimycin A together with rotenone after the ninth measuring point is shown. From this curve, various parameters can be calculated, which are marked in different colors. The calculations of the parameters are shown on the right side.
The protocol described in detail hereafter has been applied to examine the influence of 12 days of in vivo caloric reduction in humans. A significant increase of mitochondrial respiratory parameters could be detected in PBMCs of a subgroup of the test persons (Schöller-Mann et al., 2020). Besides such ex vivo studies the protocol can be used to screen any soluble substance, including pharmaceuticals, dietary supplements or contaminants, due to their in vitro effects on PBMCs.
Materials and Reagents
15 ml, 50 ml screw cap tubes (SARSTEDT, catalog numbers: 62.554.502 ; 62.547.254 )
1.5 ml, 2 ml reaction tubes (SARSTEDT, catalog numbers: 72.706 ; 72.695.500 )
20 µl, 200 µl, 1,000 µl pipette tips (SARSTEDT, catalog numbers: 70.1116 ; 70.760.002 ; 70.762 )
5 ml, 10 ml, 25 ml serological pipettes (SARSTEDT, catalog numbers: 86.1253.001 ; 86.1254.001 ; 86.1685.001 )
50 ml LeucosepTM tubes (Greiner Bio-One GmbH, catalog number: 227290 )
Seahorse XF24 FluxPak containing XF24 sensor cartridges, XF24 cell culture microplates and XF Calibrant Solution (Agilent Technologies, catalog number: 100850-001 )
Human venous blood, EDTA-anticoagulated – S-Monovette® 7.5 ml K3E (SARSTEDT, catalog number: 01.1605.001 )
Ficoll-PaqueTM PLUS (GE Healthcare, catalog number: 17-1440-03 )
Poly-D-Lysine solution, 1.0 mg/ml (Merck Millipore, catalog number: A-003-E , storage temp. -20 °C)
Trypan Blue solution 0.4% (Sigma-Aldrich, catalog number: 93595 )
Dulbecco’s Modified Eagle’s Medium (DMEM), high glucose (Sigma-Aldrich, catalog number: D7777 , storage temp. 4 °C)
Dimethyl sulfoxide (DMSO) (Carl Roth, catalog number: A994 )
Oligomycin from Streptomyces diastatochromogenes (Sigma-Aldrich, catalog number: O4876 , storage temp. -20 °C; mixture of isomers A, B, and C), 2.5 mM in DMSO
Carbonyl cyanide 4-(trifluoromethoxy) phenylhydrazone (FCCP; Sigma-Aldrich, catalog number: C2920 , storage temp. 4 °C), 2.5 mM in DMSO
Rotenone (Sigma-Aldrich, catalog number: R8875 ), 2.5 mM in DMSO
Antimycin A from Streptomyces sp. (Sigma-Aldrich, catalog number: A8674 , storage temp. -20 °C), 2.5 mM in DMSO
NaCl (Carl Roth, catalog number: 3957 )
NaOH (Carl Roth, catalog number: 6771 )
KCl (Carl Roth, catalog number: 6781 )
Na2HPO4·12H2O (Carl Roth, catalog number: N350 )
KH2PO4 (Carl Roth, catalog number: 3904 )
1× PBS (see Recipes)
0.9% NaCl solution (see Recipes)
10 M NaOH solution (see Recipes)
Poly-D-Lysine working solution (50 µg/ml) (see Recipes)
2.5 mM oligomycin solution (see Recipes)
2.5 mM FCCP solution (see Recipes)
0.1 M rotenone solution (see Recipes)
2.5 mM rotenone solution (see Recipes)
2.5 mM antimycin A solution (see Recipes)
Assay medium (see Recipes)
Equipment
Pipettes: Eppendorf Research® Plus 10 μl, 20 µl, 200 µl, 1,000 µl (Eppendorf, catalog numbers: 3123000020 ; 3123000039 ; 3123000055 ; 3123000063 )
Pipetting aid: PipetBoy acu 2 (Integra Biosciences, catalog number: 155 000)
Neubauer counting chamber improved (Carl Roth, catalog number: PC72.1 )
Inverted microscope: Primovert (Carl Zeiss, catalog number: 415510-1100-000 )
Water bath (GFL, catalog number: 1003 )
Incubator without CO2 (GFL, catalog number: 4010 )
Swing out rotor centrifuge: 5804 R (Eppendorf, catalog numbers: 5805000010 ; 5804709004 )
Water purification system: ELGA® PURELAB flex 3 (Veolia, catalog number: PF3XXXXM1 )
pH meter: FE20 FiveEasyTM (Mettler Toledo, cataog number: 30266626 )
Seahorse XF24 Extracellular Flux Analyzer (Agilent Technologies, catalog number: 100737-100 )
Software
Wave Controller Software (Agilent Technologies, version 1.8.1.1)
Optional: Wave Desktop Software (Agilent Technologies)
Procedure
文章信息
版权信息
© 2021 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Meßmer, A. L., Matt, K. C., Hochecker, B. and Bergemann, J. (2021). Ex vivo Assessment of Mitochondrial Function in Human Peripheral Blood Mononuclear Cells Using XF Analyzer. Bio-protocol 11(7): e3980. DOI: 10.21769/BioProtoc.3980.
分类
免疫学 > 免疫细胞功能 > 淋巴细胞
细胞生物学 > 基于细胞的分析方法 > 线粒体呼吸
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link










