- EN - English
- CN - 中文
Monitoring Real-time Temperature Dynamics of a Short RNA Hairpin Using Förster Resonance Energy Transfer and Circular Dichroism
利用荧光共振能量转移和圆二色性监测短发夹RNA的实时温度动态
发布: 2021年03月20日第11卷第6期 DOI: 10.21769/BioProtoc.3950 浏览次数: 5297
评审: Wenrong HeYingnan HouLusheng Fan

相关实验方案

Turbo-RIP:基于 TurboID 的 RNA 免疫纯化方法,用于绘制植物生物分子凝聚体中的 RNA 图谱
Zhi Zhang [...] Panagiotis Nikolaou Moschou
2026年02月05日 331 阅读
Abstract
RNA secondary structures are highly dynamic and subject to prompt changes in response to the environment. Temperature in particular has a strong impact on RNA structural conformation, and temperature-sensitive RNA hairpin structures have been exploited by multiple organisms to modify the rate of translation in response to temperature changes. Observing RNA structural changes in real-time over a range of temperatures is therefore highly desirable. A variety of approaches exists that probe RNA secondary structures, but many of these either require large amount and/or extensive processing of the RNA or cannot be applied under physiological conditions, rendering the observation of structural dynamics over a range of temperatures difficult. Here, we describe the use of a dually fluorescently labelled RNA oligonucleotide (containing the predicted hairpin structure) that can be used to monitor subtle RNA-structural dynamics by Förster Resonance Energy Transfer (FRET) at different temperatures with RNA concentration as low as 200 nM. FRET efficiency varies as a function of the fluorophores’ distance; high efficiency can thus be correlated to a stable hairpin structure, whilst a reduction in FRET efficiency reflects a partial opening of the hairpin or a destabilisation of this structure. The same RNA sequence can also be used for Circular Dichroism spectroscopy to observe global changes of RNA secondary structure at a given temperature. The combination of these approaches allowed us to monitor RNA structural dynamics over a range of temperatures in real-time and correlate structural changes to plant biology phenotypes.
Graphic abstract:
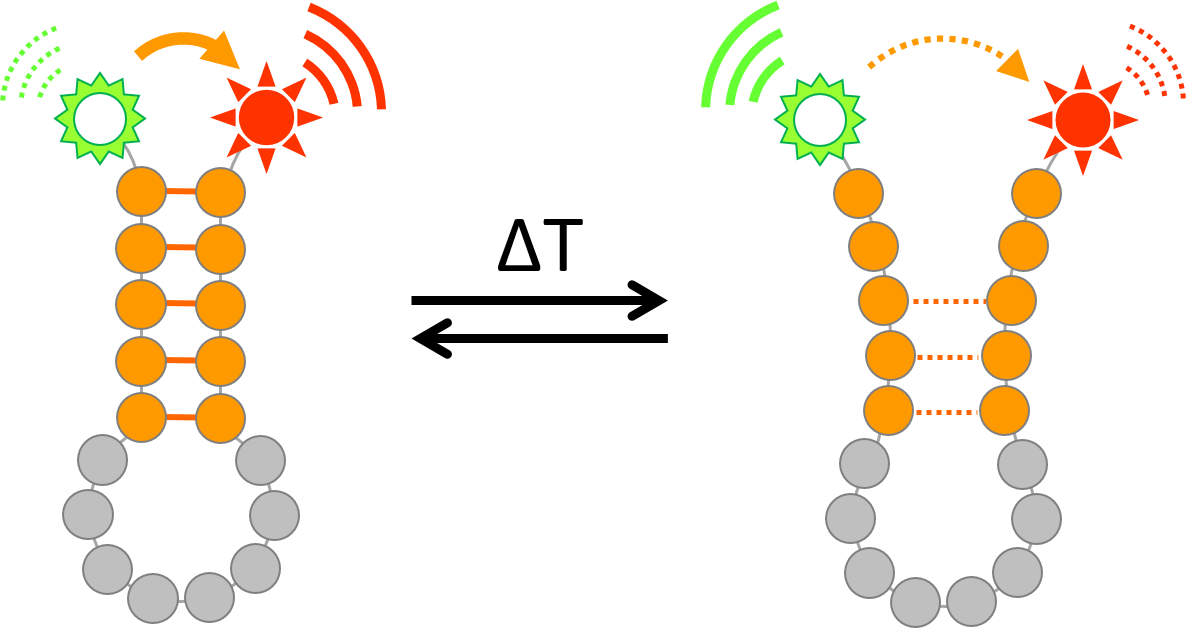
Monitoring temperature-dependent RNA structural dynamics using Förster Resonance Energy Transfer (FRET)
Background
RNA is the central molecule in gene expression in all extant life, serving as the messenger. It is also central to biocatalysis, playing a key role in ribosome assembly and function. Ultimately, RNA function is regulated by its structure and there is a seemingly limitless variety of structures that allows for diverse functions. RNA molecules can form both inter- and intramolecular Watson-Crick and Hoogsteen base pairs, which lead to the formation of secondary structures such as hairpins, apical and internal loops, 3-way-junctions or pseudoknots (Cruz and Westhof, 2009). Such secondary structures can impart catalytic activity, serve as molecular scaffolds as well as provide or occlude binding sites for RNA-binding proteins (RBPs) and other RNAs. Secondary structures on messenger RNA are known to affect a plethora of biological processes such as RNA stability and processing (Soemedi et al., 2017), alternative splicing (Bartys et al., 2019), subcellular localisation (Lazzaretti and Bono, 2017) and translation regulation (Jacobs et al., 2012).
Whilst RNA molecules have the capacity to adopt a multitude of distinct conformations, the most thermodynamically and kinetically favourable structures will be populated under certain conditions. As a consequence, RNA secondary structures can be highly dynamic and interconvert between different structural arrangements as the environment changes (Ganser et al., 2019; Mustoe et al., 2014). For example, binding of RBPs can induce or facilitate structural rearrangement, while riboswitches change their conformation and exert regulatory function upon binding of small ligands. Finally, physical parameters such as temperature and osmolarity strongly affect the distribution and the stability of RNA secondary structures and thereby promote structural transitions. Given the biological relevance of RNA secondary structure formation, several biochemical and biophysical strategies have been developed to monitor RNA structural changes in real-time, including Circular Dichroism, NMR, DMS-footprinting, and Förster Resonance Energy Transfer (FRET).
As RNA secondary structures are dependent on their thermodynamic free energies, temperature is one of the most direct parameters to control such structures for translation regulation. Thermosensitive RNA secondary structures – referred to as RNA thermometers – have been harnessed by multiple organisms to fine-tune translation in a temperature-dependent manner (Kortmann and Narberhaus, 2012; Krajewski and Narberhaus, 2014). A classic example of an RNA thermometer is found in Listeria, where a secondary structure in the prfA transcript controls virulence in response to temperature: At temperatures up to 30 °C, a hairpin structure forms and prevents access to the ribosome binding site; this structure partially melts when temperature rises to 37 °C (the body temperature of the human host), exposing the ribosome binding site and allowing translation to occur (Johansson et al., 2002). Many prokaryotes employ such zipper-like RNA thermometers as a switch to control translation. However, increased temperature may not necessarily lead to a complete loss of secondary structure, but favour the adoption of alternative conformations instead. We recently identified several eukaryotic RNA thermoswitches in the model flowering plant Arabidopsis thaliana, which are activated at warm temperatures (27-32 °C) by adopting a more relaxed hairpin conformation to enhance translation (Chung et al., 2020).
These examples emphasise the importance of investigating RNA structural dynamics in order to rationalize their biological functions. Chemicals or enzymes that react selectively with single or double stranded RNA can be employed to probe secondary structure both in vitro and in vivo (Mailler et al., 2019). However, these approaches are irreversible, leaving the RNA molecules permanently modified and preventing real-time observation of the same molecules’ dynamics over a range of conditions. Several biophysical methods can be used to observe RNA secondary structure as well, but come with their own set of limitations: besides significant requirement of RNA input, nuclear magnetic resonance (NMR) spectroscopy and X-ray crystallography preclude observation under physiological conditions, while cryo electron microscopy still suffers from limited resolution and is thus currently restricted to larger structures (Mailler et al., 2019). In our characterisation of plant RNA thermoswitches, we employed a combination of Förster Resonance Energy Transfer (FRET) measurements and circular dichroism (CD) spectroscopy to observe RNA hairpin dynamics over a range of temperatures in real-time. A detailed protocol of these methods is outlined below.
Materials and Reagents
1.5 ml Eppendorf tube
Semi-micro quartz cell 108F-QS (Sigma-Aldrich, Hellma Analytics, catalog number: HL108-F-10-40 )
1 mm quartz cell 350 ml (Sigma-Aldrich, Hellma Analytics, catalog number: HL110-F-1-40 )
Cacodylic Acid (Sigma-Aldrich, catalog number: C0125-5G ). Store at 4 °C
KOH in pellets, BioXtra (Sigma-Aldrich, catalog number: P5958 )
RNase-free water (Sigma-Aldrich, catalog number: W4502 )
RNA oligonucleotide with 5’-FAM and 3’-TAMRA fluorescent probes (5’-FAM-AAG AGA GCU UAA UUG UCA GUU UAU UCU CUG-3’-TAMRA in case of the PIF7 hairpin); the RNA sequence should cover the entire predicted hairpin-forming sequence, but should not exceed more than 3 nucleotides beyond this sequence on either side to ensure efficient FRET (IBA Gmbh, HPLC Purified). Store at 4 °C
Cacodylate buffer (see Recipes)
1 M KOH (see Recipes)
Equipment
2 L glass beaker
Stirring bar
SevenCompact pH meter S220 (Mettler Toledo, model: 30019029 )
-80 °C freezer
Varian Cary Eclipse Fluorescence Spectrometer fitted with a Varian temperature control unit (Agilent, Varian, Cary Eclipse Fluorimiter)
ThermoMixer C (Eppendorf, model: EP5382000015 ) fitted with SmartBlock 1.5 ml (Eppendorf, model: EP5361000031 )
Applied Photophysics Chirascan (CD) fitted with temperature control unit (Applied Photophysics, ChirascanTM)
Software
Cary Eclipse Kinetics software (Agilent, https://www.agilent.com/en/product/molecular-spectroscopy/fluorescence-spectroscopy/fluorescence-software/cary-eclipse-software)
GraphPad Prism 8 (GraphPad Software, https://www.graphpad.com/scientific-software/prism/)
Procedure
文章信息
版权信息
© 2021 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Balcerowicz, M., Di Antonio, M. and Chung, B. Y. W. (2021). Monitoring Real-time Temperature Dynamics of a Short RNA Hairpin Using Förster Resonance Energy Transfer and Circular Dichroism. Bio-protocol 11(6): e3950. DOI: 10.21769/BioProtoc.3950.
分类
生物化学 > RNA > RNA结构
植物科学 > 植物分子生物学 > RNA
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link











