- EN - English
- CN - 中文
A Spectrofluorophotometrical Method Based on Fura-2-AM Probe to Determine Cytosolic Ca2+ Level in Pseudomonas syringae Complex Bacterial Cells
基于Fura-2-AM探针的荧光光度法测定丁香假单胞菌复合菌胞内Ca2+水平
发布: 2021年03月20日第11卷第6期 DOI: 10.21769/BioProtoc.3949 浏览次数: 5301
评审: Delfina C DominguezDhaneswar PrustyAnonymous reviewer(s)

相关实验方案

通过制备连续聚丙烯酰胺凝胶电泳和凝胶酶谱分析法纯化来自梭状龋齿螺旋体的天然Dentilisin复合物及其功能分析
Pachiyappan Kamarajan [...] Yvonne L. Kapila
2024年04月05日 2095 阅读
Abstract
Calcium signaling is an emerging mechanism by which bacteria respond to environmental cues. To measure the intracellular free-calcium concentration in bacterial cells, [Ca2+]i, a simple spectrofluorometric method based on the chemical probe Fura 2-acetoxy methyl ester (Fura 2-AM) is here presented using Pseudomonad bacterial cells. This is an alternative and quantitative method that can be completed in a short period of time with low costs, and it does not require the induction of heterologously expressed protein-based probes like Aequorin. Furthermore, it is possible to verify the properties of membrane channels involved in Ca2+ entry from the extracellular matrix. This method is in particular valuable for measuring [Ca2+]i in the range of 0.1-39.8 µM in small cells like those of prokaryotes.
Keywords: Fura 2-AM (Fura2-乙酰氧基甲酯)Background
Ca2+ is an emerging intracellular messenger of bacteria that impacts a wide array of cellular processes such as the maintenance of cell integrity, cell division (Dominguez et al., 2015), motility (Tisa and Alder, 1995; Gode-Potratz et al., 2010; Cruz et al., 2012; Guragain et al., 2013; Parker et al., 2015; Fishman et al., 2018), type III secretion (DeBord et al., 2003; Dasgupta et al., 2006; Gode-Potratz et al., 2010; Fishman et al., 2018), gene expression (Dominguez et al., 2015), quorum sensing (Werthén and Lundgren, 2001), biofilm formation (Patrauchan et al., 2005; Sarkisova et al., 2005; Rinaudi et al., 2006; Cruz et al., 2012; Das et al., 2014; Zhou et al., 2014; Parker et al., 2016) or biofilm suppression (Bilecen and Yildiz, 2009; Shukla and Rao, 2013). Recently, it was demonstrated that the intracellular Ca2+ concentration controls virulence of Pseudomonas savastanoi pv. savastanoi (Psav) (Moretti et al., 2019). Furthermore, several known virulence genes were upregulated in the presence of increasing Ca2+ concentrations in Pseudomonas syringae pv. tomato (Pto) DC3000 (Fishman et al., 2018), and Xylella fastidiosa (Parker et al., 2016). Measurement of the intracellular free-calcium concentration, [Ca2+]i, in prokaryotes has, therefore, become of great interest to study its role as intracellular messenger of bacteria in response to environmental cues. Monitoring the [Ca2+]i within bacterial cells, which is indispensable for understanding the correlations between the transport of Ca2+ across the plasma membrane and cellular processes was thus far difficult, as it was hampered by the small size of the bacterial cells, the semi-selective nature of the bacterial cell wall, the low membrane permeability, and the toxicity of many Ca2+ chelators used (Gangola and Rosen, 1987; Knight et al., 1991; Futsaether and Johnsson, 1994; Norris et al., 1996; Herbaud et al., 1998; Jones et al.,1999; Torrecilla et al., 2001). A well-established method to determine changes in the [Ca2+]i in prokaryotes is based on the heterologous expression of Aequorin (a calcium-activated photoprotein) bacterial cells (Watkins et al., 1995). This method employs the expression of recombinant Aequorin (from a plasmid or integrated in the bacterial genome), which emits light upon Ca2+ binding. This method is rather time-consuming, requires the availability of molecular biology tools, and is technically challenging. In fact, the method is better suited for eukaryotic cells even if it was successfully used to monitor [Ca2+]i in several bacterial species (Naseem et al., 2007; Guragain et al., 2016). To overcome these limitations of Aequorin, we developed an alternative and complementary spectrofluorometric method based on the chemical probe Fura 2-AM {1-[2-(5-carboxyoxazol-2-yl)-6-amino-benzofuran-5-oxy]-2-(2’-amino-5’-methylphenoxy) ethan-N,N,N’,N’-tetraacetic acid}. Importantly, both the assay solution used and the Fura 2-AM do not compromise cell viability at the concentrations here used (Gangola and Rosen, 1987; Futsaether and Johnsson, 1994; Tisa and Alder, 1995; Norris et al., 1996; Jones et al., 1999), meaning that this method allows quantification of [Ca2+]i in response to external cues and different conditions without the need of advanced equipment (Moretti et al., 2019). It must be pointed out that Fura 2-AM is a probe that diffuses across the cell membrane of viable bacterial cells and its subsequent rapid de-esterification by cellular esterases yields Fura 2, which retains the ability to bind the cytosolic Ca2+ while it losses the ability to diffuse across the cell membrane (Figure 1). When Fura 2 forms a complex with Ca2+, the intensity of the fluorescence at λ=510 nm increases with increasing Ca2+ concentration (Grynkiewicz et al., 1985) (Figure 2). In addition, Fura 2 is unable to permeate bacterial cells itself due to the selective permeability of the cell walls and membranes (Grynkiewicz et al., 1985). Of notice, since the measurements make use of a fluorescence signal that only becomes apparent inside cells (when Fura 2-AM is converted to Fura 2), it is not necessary to use unloaded viable cells as negative control. In fact, if the cells are not viable, the cell membrane loses its integrity and the probe would not be trapped in the cells but rather remain dispersed in the incubation medium.
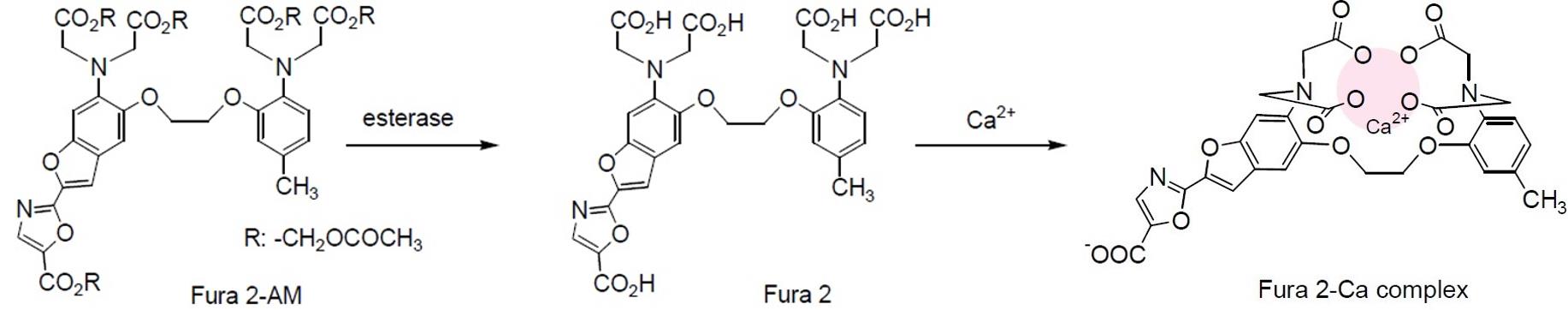
Figure 1. The fate of Fura 2-AM in cells. Fura 2-AM is de-esterified by cellular esterases and transformed in Fura 2, which is able to form a complex with cytosolic calcium (Ca2+) and cannot passively cross the cell membrane.
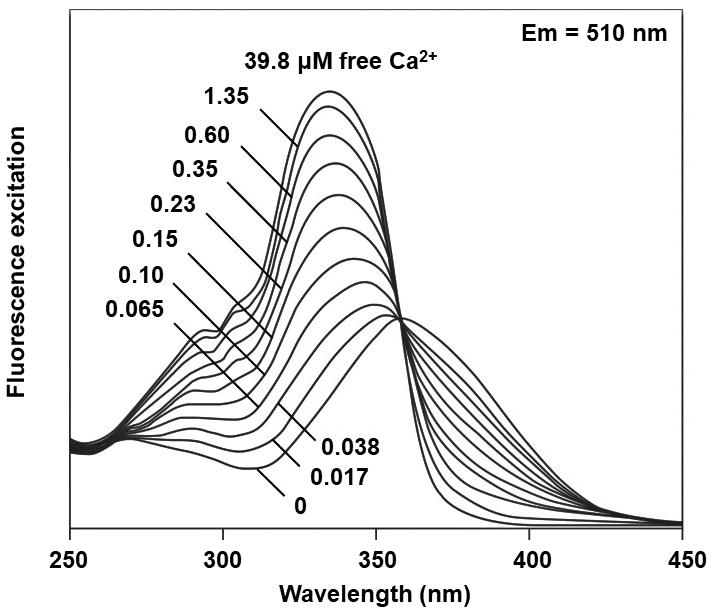
Figure 2. Excitation spectra of Fura 2. Excitation spectra of Fura 2 in solution containing 0 to 39.8 µM of free calcium (Ca2+). Modified from Grynkiewicz et al. (1985).
We find that the fluorescent probe Fura 2-AM is highly sensitive allowing us to determine changes in cytosolic Ca2+ levels in Pseudomonas savastanoi pv. savastanoi DAPP-PG 722 (Moretti et al., 2019) and Pseudomonas syringae pv. tomato DC3000 (Trabalza et al., in preparation) cells by using a spectrofluorometer equipped with a stirred semi-micro cuvette.
Materials and Reagents
Inoculation loops 10 µl (Laboindustria S.P.A., catalog number: 21131 )
Petri dish Ø 90 (Laboindustria S.P.A., catalog number: 21050 )
Pipette tips (Mettler Toledo, catalog numbers: 17007956, 17007952 )
High clarity polypropylene (PP) conical centrifuge tube 50 ml (Falcon, catalog number: 352070 )
NIR Quartz SUPRASIL 300 Rectangular Macro Cell with Lid, volume 3.5 ml (PerkinElmer, catalog number: B0631015 )
PIREX Media bottles, graduated, Corning, 500 ml (VWR, PIREX, catalog number: 1395-500 )
Pseudomonas savastanoi pv. savastanoi (Psav) DAPP-PG 722 strain (Moretti et al., 2014), stored at -80 °C in 15% glycerol
Pseudomonas syringae pv. tomato (Pto) DC3000 strain (Gizjen, 2008), stored at -80 °C in 15% glycerol
MilliQ double distilled water
Tris base (Sigma-Aldrich, catalog number: T1503 ); prepare 0.12 M aqueous solution, adjust pH to 8.0 with 1 M HCl, autoclave and store at room temperature
Fura 2-AM (Sigma-Aldrich, catalog number: F0888 ); prepare 2 mM solution in DMSO in aliquots of 50 µl and freeze at -20 °C. Store at -20 °C, wrap it in aluminum foil to avoid photodegradation. Avoid repeated freezing and thawing of the aliquots
EGTA (Sigma-Aldrich, catalog number: E3889 ); prepare a 0.5 M aqueous stock solution, adjust pH to 8.0 with 0.5 M NaOH, autoclave and store at room temperature. Prepare a 2 mM aqueous solution from the stock solution
Sodium chloride (Sigma-Aldrich, catalog number: S7653 )
Tryptone (Sigma-Aldrich, Millipore, catalog number: T7293 )
Yeast extract (Sigma-Aldrich, catalog number: Y1625 )
Calcium chloride (Sigma-Aldrich, catalog number: 449709 ); prepare a 50 mM aqueous solution and autoclave at 121 °C for 20 min
Hank’s Buffered Salt Solution (HBSS) buffer; prepare 1 L aqueous solution with 8.18 g/L NaCl, 0.4 g/L KCl (Sigma-Aldrich, catalog number: P9541 ), 5.96 g/L HEPES (Sigma-Aldrich, catalog number: H3375 ), adjust pH to 7.4 and autoclave at 121 °C for 20 min
EDTA (Sigma-Aldrich, catalog number: E9884 ); prepare a 0.5 M aqueous stock solution, adjust pH to 8.0 with NaOH, autoclave at 121 °C for 20 min and store at room temperature. Prepare a 0.1 mM aqueous solution from the stock solution
Triton X-100 (Sigma-Aldrich, catalog number: X100 ); prepare a 1% aqueous solution and autoclave at 121 °C for 20 min
Luria Bertani (LB) medium (see Recipes)
Equipment
1 L measuring cylinder (DWK Life Sciences, catalog number: 21 390 54 08 )
BRAND magnetic stirring bar (Sigma-Aldrich, Aldrich, catalog number: BR137630 )
Magnetic stirrer (Heidolph MR 2000, catalog number: 200-505-20000-00 )
Eppendorf Research Plus G pippetes (Sigma-Aldrich, Eppendorf, catalog number: EP3123000918 )
Autoclave (EXAPro, Lequeux, catalog number: P80602001 )
Shaking incubator SI500 (Stuart, catalog number: FV-79520-00 )
Eppendorf® Centrifuge 5804R (Sigma-Aldrich, Sigma, catalog number: EP022628146 )
HerathermTM Incubator (Thermo Scientific, catalog number: 51028112 )
LS-50B Luminescence Spectrometer (PerkinElmer, catalog number: 17931 )
Laminar flow cabinet Gelaire BSB 6A (Gelaire)
Software
FL WinLab Software, version 3 (PerkinElmer)
Prism 8 (GraphPad, https://www.graphpad.com/scientific-software/prism/)
Procedure
文章信息
版权信息
© 2021 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Trabalza, S., Buonaurio, R., Del Pino, A. M., Palmerini, C. A., van den burg, H. A. and Moretti, C. (2021). A Spectrofluorophotometrical Method Based on Fura-2-AM Probe to Determine Cytosolic Ca2+ Level in Pseudomonas syringae Complex Bacterial Cells. Bio-protocol 11(6): e3949. DOI: 10.21769/BioProtoc.3949.
分类
微生物学 > 微生物-宿主相互作用 > 细菌
细胞生物学 > 基于细胞的分析方法 > 钙离子稳态
植物科学 > 植物免疫 > 宿主-细菌相互作用
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link











