- EN - English
- CN - 中文
Defined Mutant Library Sequencing (DML-Seq) for Identification of Conditional Essential Genes
确定突变体文库测序(DML-Seq)用于鉴定的条件必需基因
(*contributed equally to this work) 发布: 2021年03月05日第11卷第5期 DOI: 10.21769/BioProtoc.3943 浏览次数: 4616
评审: Srujana Samhita YadavalliThirugnanasambandam RajendranAnonymous reviewer(s)
Abstract
Transposon insertion sequencing (TIS) is an emerging technique which utilizes a massive transposon mutant library to screen specific phenotype and determine the conditional essential genetic requirements for bacterial fitness under distinct conditions combined with high-throughput parallel sequencing technology. Compared with a massive mutant library in traditional TIS, the defined mutant library sequencing (DML-Seq) has advantages as: 1) efficient mutagenesis; 2) low bottleneck effects; 3) avoid hotpots caused by screening; 4) can be directly used in the following experiments. Here, we described an optimized procedure of DML-Seq for fitness screen to supply classical TIS using the marine pathogenic bacterium Edwardsiella piscicida as an example.
Keywords: TIS (转座子插入测序)Background
Transposon insertion mutagenesis coupled with next-generation sequencing (NGS) has been proven to be a powerful method to investigate gene function under a multitude of conditions (Chao et al., 2016; Price et al., 2018). In general, TIS analysis consists of the massive parallel sequencing of transposon insertion sites and statistical analysis of the abundance of the insertion events.
TIS-based screens can provide high-resolution maps of the fitness contributions of individual loci and domains based on the bacterial fitness of highly saturated transposon mutant libraries under a multitude of conditions (Chao et al., 2016). The insertion frequency at each locus or the relative abundance of corresponding mutants is generally inversely correlated with the locus’s contribution to fitness following the imposition of a selective pressure, such as that imposed by hosts and antibiotics (Chao et al., 2016). The principles of this methodology underlie a variety of related approaches, including TIS, transposon sequencing (TnSeq), insertion sequencing (INSeq), transposon-directed insertion site sequencing (TraDIS), high-throughput insertion tracking by deep sequencing (HITS), and transposon insertion site sequencing (TIS-Seq) (Gawronski et al., 2009; Langridge et al., 2009; Chao et al., 2016; Veeranagouda et al., 2017; Price et al., 2018). These approaches are revolutionizing microbiological studies by facilitating unprecedented and deep genome-wide explorations of a wide range of bacterial species.
With the aim to generate highly saturated mutant libraries for further analysis, the insertion events caused by transposon should be unbiased and the transposon will be delivered into recipient cells several times. However, this will lead to a large amount of nonsense mutation and the complexity of mutant libraries may cause bottleneck effect during the following screens. Compared with this, the defined mutant libraries containing mutants with a single insertion at a known genomic location will be more useful and facilitated (Fu et al., 2013; Abel et al., 2015). The first human pathogenic bacterium defined mutant library for Pseudomonas aeruginosa was constructed in 2003. Subsequently, many defined mutant libraries of human pathogens were also generated, such as a library of Francisella tularensis in 2007, Vibrio cholera in 2008, Burkholderia thailandensis in 2013, Chlamydia and Mycobacterium in 2015 (Jacobs et al., 2003; Gallagher et al., 2007 and 2013; Cameron et al., 2008; Kokes et al., 2015). With these defined mutant libraries, a myriad of important researches were completed, such as the identification of ciprofloxacin resistome from P. aeruginosa library, a novel T6SS regulator TsrA from V. cholera library, and the exploration of functional genomics with Desulfovibrio alaskensis G20 library (Breidenstein et al., 2008; Zheng et al., 2008 and 2010; Kuehl et al., 2014). Hence, such defined libraries may serve as valuable resources because they often consist of collections of insertion mutants in almost all non-essential loci for an organism of interest and have drawn more attention to the exploration of functional genomics of microbe.
In order to optimize TIS approach, we developed the DML-seq method which adopts the mixed defined mutant libraries for conditional essential fitness screenings (Figure 1) (Wei et al., 2019a and 2019b). Compared with massive mutant library in traditional TIS, the DML library has advantages as: 1) insertions within the 80% of the ORF to ensure the effective mutagenesis; 2) low complexity and few isogenes to ensure low bottleneck effects; 3) the mixture of an equal amount of individual mutants to avoid hotpots caused by screening; 4) can be directly used in the following experiments.
Compared to human pathogenic bacterium, the pathogenesis of marine pathogenic bacterium is poorly understood. In our model organism Edwardsiella piscicida, which causes Edwardsiellosis in farmed fish and leads to severe economic losses in aquaculture worldwide (Leung et al., 2019), DML-seq has been employed to identify conditional essential genes as well as those crucial for survival in different environments (Wei et al., 2019b), colonization and infection in host cells (Wei et al., 2019a). Briefly, we constructed MKGR, a derivative of Himar1 mariner transposon based on MAR2xT7 (Liberati et al., 2006), to generate a library of random transposon insertion mutants of E. piscicida EIB202 (Table 1). In total, 2,806 genes were disrupted out of the 3,599 predicted genes with an average of 5.04-fold coverage on E. piscicida chromosome. After that, 5 non-redundant subset libraries were created manually. The 1st and 2nd subset libraries contained a single mutant for each disrupted ORF in the parental library and they were paralleled for each other. The 3rd subset library contains transcriptional fusion mutants to eliminate the location effect on transcription. The 4th subset library is an intergenic library and the 5th library was a composited library, which was generated by mixing the 1st, 2nd, 4th subset libraries into a pool.
Here, we will focus on the specific steps for DML-seq because the excellent protocols deTAILing TIS analyses are reviewed and available elsewhere (Chao et al., 2016; Yamaichi et al., 2017). In principle, the procedure described here can be applied to conditional essential screens and facilitate the exploration of functional genomics of microbe.
Table 1. The statistic values of defined mutant library of E. piscicida EIB202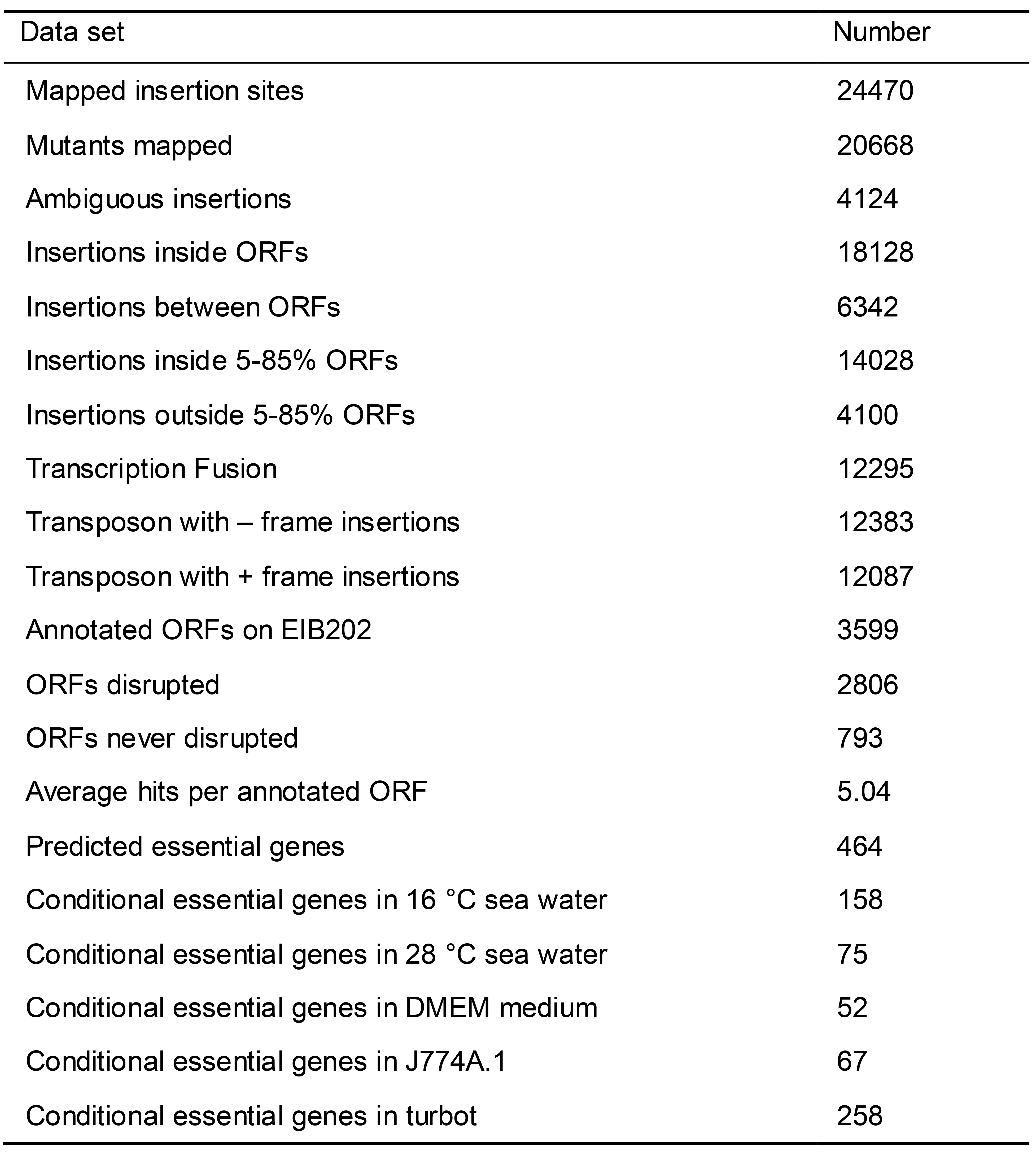

Figure 1. A defined transposon mutant library construction. A. pMKGR, a derivative of Himar1 mariner transposon, was constructed to build the defined transposon mutant library of E. piscicida EIB202. B. Work flow of a defined mutant library construction. E. coli SM10 is used as a donor for conjugation with recipient strain E. piscicida EIB202. Conjugation is processed between EIB202 and SM10 on LB plates for 3 h at 30 °C. Later, conjugators are separated on LB plates containing 20 µg/ml polymyxin and 15 µg/ml gentamicin. After 24 h of incubation at 30 °C, monoclonal will be picked into 96-well plates and incubated at 30 °C for about 16 h. Before storing at -80 °C, glycerol was added into every well and mix to make the final 20% glycerol concentration to preserve strains. C. Schematic of TAIL-PCR amplification used for determination of the Tn insertion loci on the genome. The pair of primer Sp1/AB2 is used for the first round of PCR and another pair of primer Sp2/ABS is used for the second round of PCR. Finally, primer Seq2 is used for sequencing of the amplified PCR products. D. The number of new disrupted genes increased linearly with new mutants and the number of genes hit approached a plateau.
Materials and Reagents
Handling Bacteria
Large square Petri dish (500 cm2) (Thermo Fisher Scientific, catalog number: R80115TS )
Cellulose filter membrane (0.45 μm pore size, Merck Millipore)
Eppendorf tube (Axygen, catalog number: MCT-150-C )
PCR tube (Axygen, catalog number: PCR-02-A )
Corning 50 ml centrifuge tube (Merck, catalog number: CLS430828-500EA )
96-Well Deep Well Plates (Thermo Fisher Scientific, catalog number: A43075 )
Cryotube (Thermo Fisher Scientific, catalog number: 5000-1020 )
Wild type E. piscicida EIB202 (ColR); “recipient” cells
E. coli SM10 λpir harboring pMKGR for delivering Tn via conjugation (AmpR, KmR); “donor” cells (see Note 1)
Luria-Bertani (Miller's LB Broth Base, Thermo Fisher Scientific, catalog number: 12795027 ) and agar media (Merck, catalog number: 05040 )
Colistin (Col, 20 mg/ml, BBI)
Ampicillin (Amp, 100 mg/ml, BBI)
Gentamicin (Gm, 15 mg/ml, BBI)
Glycerol (Titan, catalog number: 56-81-5 )
Thermal Asymmetric Interlaced PCR (TAIL-PCR)
Genomic DNA (gDNA) Purification
Library Construction
Qiagen Gel purification kit (QIAGEN, catalog number: 28104 )
VAHTS HiFi Amplification Mix (Vazyme Biotech, catalog number: 616-01/02 )
Agarose (Thermo Fisher Scientific, catalog number: 16500500 ) and Electrophoresis apparatus (BIO-RAD, catalog number: 1704489EDU )
QubitTM dsDNA HS Assay kit (Invitrogen, catalog number: Q32851 )
Oligo DNAs (See Note 2) (HPLC purified, BGI)
“EIB202-F”: 5’-TCATCGCACATACAGAATAAACGCC-3’
“EIB202-R”: 5’-CCGTAACATTTCTTACAACACTGCG-3’
“pMKGR-F”: 5’-AGGTGATGCTACATACGGAAAG-3’
“pMKGR-R”: 5’-AGCGCATGAACTCCTTGATG-3’
“Tn-F”: 5’-AAAAGTCCGCTGGCAAAG-3’
“Tn-R”: 5’-CCCTTCAAGAGCGATACAAC-3’
“Sp1-F”: 5’-GCTCCGTAGTAAGACATTCATCGCG-3’
“Sp2-F”: 5’-GCTTACGTTCTGCCCAAGTTTGAG-3’
“ABS-R”: 5’-GGCCACGCGTCGACTAGTAC-3’
“AB2-R”: 5’-GGCCACGCGTCGACTAGTACNNNNNNNNNNCCTGG-3’
“Seq2-F”: 5’-CAATTCGTTCAAGCCGAGATCG-3’
“AD_fork truncated NH2”: 5’- TACCACGACCA-3’
“AD_Index Fork R”: 5’- GTGACTGGAGTTCAGACGTGTGCTCTTCCGATCTATGGTCGTGGT-3’
“1st_seq-out-psc189”: 5’- ACTCTGGGGTACGCGTCTAG-3’
“1st_PCR_Index ‘R’ primer”: 5’-GTGACTGGAGTTCAGACGTGTG-3’
“P5 spacer primers”: equimolar mixture of the following 6 oligos:
5’-AATGATACGGCGACCACCGAGATCTACACTCTTTCCCTACACGACGCTCTTCCGATCTGACTTATCAGCCAACCTGT-3’
5’-AATGATACGGCGACCACCGAGATCTACACTCTTTCCCTACACGACGCTCTTCCGATCTCGACTTATCAGCCAACCTGT-3’
5’-AATGATACGGCGACCACCGAGATCTACACTCTTTCCCTACACGACGCTCTTCCGATCTATGACTTATCAGCCAACCTGT-3’
5’-AATGATACGGCGACCACCGAGATCTACACTCTTTCCCTACACGACGCTCTTCCGATCTTGTCGACTTATCAGCCAACCTGT-3’
5’-AATGATACGGCGACCACCGAGATCTACACTCTTTCCCTACACGACGCTCTTCCGATCTTCGACGACTTATCAGCCAACCTGT-3’
5’-AATGATACGGCGACCACCGAGATCTACACTCTTTCCCTACACGACGCTCTTCCGATCTGCAGCGACGACTTATCAGCCAACCTGT-3’
“P7 barcode primers”
CAAGCAGAAGACGGCATACGAGATNNNNNNGTGACTGGAGTTCAGACGTGTGCTCTTCCGATC (vary from sample to sample)
Equipment
Multichannel pipette (Thermo Fisher Scientific)
High speed centrifuge (Eppendorf, model: 5415R )
Thermal cycler (EASTWIN, model: ETC811 )
Water bath (Thermo Fisher Scientific, catalog number: TSGP02 )
High speed centrifuge (Eppendorf, model: 5415R )
Bath sonicator (Diagenode, catalog number: B01020001 )
Thermal cycler (EASTWIN, catalog number: ETC811 )
VAHTS Universal End preparation Module for Illumina (Vazyme Biotech, catalog number: N203-01 )
VAHTS Universal Adapter Ligation Module for Illumina (Vazyme Biotech, catalog number: N204-01 )
QubitTM 4 Fluorometer (Invitrogen, catalog number: Q33238 ), or equivalent fluorescent-based DNA quantification apparatus
Procedure
文章信息
版权信息
© 2021 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Shao, S., Wei, L., Xia, F., Zhang, Y. and Wang, Q. (2021). Defined Mutant Library Sequencing (DML-Seq) for Identification of Conditional Essential Genes. Bio-protocol 11(5): e3943. DOI: 10.21769/BioProtoc.3943.
分类
分子生物学 > DNA > 诱/突变
微生物学 > 微生物-宿主相互作用 > 细菌
微生物学 > 微生物遗传学 > 诱/突变
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link