- EN - English
- CN - 中文
Simultaneous Imaging of Single Protein Size, Charge, and Binding Using A Protein Oscillation Approach
利用蛋白质振荡方法同时成像单个蛋白质的大小、电荷和结合状态
发布: 2021年03月05日第11卷第5期 DOI: 10.21769/BioProtoc.3934 浏览次数: 5165
评审: Zinan ZhouAnonymous reviewer(s)
Abstract
Electrophoresis and Western blot are important tools in protein research for detection and identification of proteins. These traditional techniques separate the proteins based on size and charge differences and identify the proteins by antibody binding. Over the past decade, the emergence of single-molecule techniques has shown great potential in improving the resolution of the traditional protein analysis methods to the single-molecule level. However, such single-molecule techniques measure either size or charge, and it is challenging to measure both at the same time. Recently, we have developed a single-molecule approach to address this problem. We tether the single proteins to a surface with a polymer linker and drive them into oscillation with an electric field. By tracking the electromechanical response of the proteins to the field using an optical imaging method, the size and charge can be obtained simultaneously. Binding of antibodies or ions to the tethered protein also changes the size and charge, which allows us to probe the interactions. This protocol includes fabrication of protein oscillators, configuration of the optical detection system, and analysis of the oscillation signal for quantification of protein size and charge. We wish this protocol will enable researchers to perform comprehensive single-protein analysis on a single platform.
Keywords: Single-molecule imaging (单分子成像)Background
Proteins play essential roles in many biological processes and serve as drug targets and biomarkers. Analysis of proteins rely on technologies including electrophoresis, Western Blot, and mass spectrometry, which separate and identify proteins based on the size and charge of protein molecules. Although powerful in detecting proteins, these technologies are not sensitive to single molecules, which is required for elucidating molecular heterogeneity and precision diagnosis. Recently, several single-molecule technologies have been developed to measure the size or charge of single biomolecules. Examples include interferometric scattering microscopy (iSCAT) (Young et al., 2018) and plasmonic scattering microscopy (PSM) (Zhang et al., 2020), which quantify the size by measuring the scattered light from the molecules. Single-molecule electrometry (Ruggeri et al., 2017) and anti-Brownian electrokinetic (ABEL) trap (Wang et al., 2012) measure the charge of single molecules by monitoring single molecule motion in a potential trap. However, simultaneously measuring the size and charge on a single platform remains challenging.
Recently, we have developed a single-molecule technique to solve this problem (Ma et al., 2020). We tether proteins to an indium tin oxide (ITO) surface by polyethylene glycol (PEG) linkers and drive them into oscillation in vertical direction by applying an alternating electric field (Figure 1A). To track the protein oscillation, we place the ITO on an inverted optical microscope and generate evanescent field on the ITO surface. The oscillating proteins scatter the evanescent field, which is collected by the microscope and detected by a CMOS camera (Figure 1B). Using this method, the oscillation can be tracked with nanometer precision. By analyzing the oscillation and electromechanical response of the proteins to the field, the size and charge of individual proteins can be determined. The current protocol describes the assembly of protein oscillators and the optical detection system. Applications including protein size, charge, and binding measurements will also be presented. This protocol is also applicable to other single-molecule detection platforms, such as PSM and iSCAT, to expand the capability in simultaneous charge measurements.
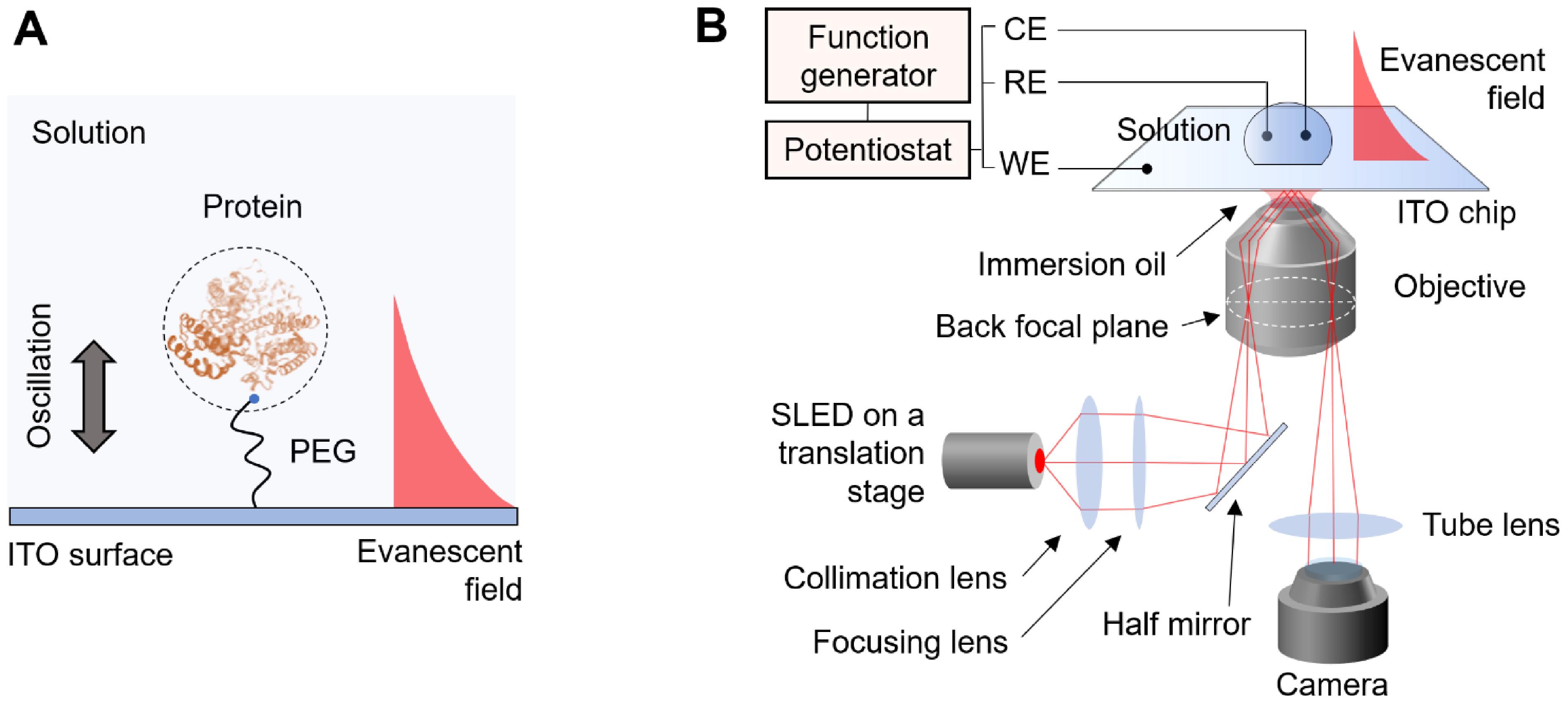
Figure 1. Detection principle and schematic of the detection system. A. The protein is tethered to the ITO surface by a PEG tether. An electric field is applied to the surface which drives the protein into oscillation. An evanescent field is generated on the surface to probe the oscillation of the protein. B. The detection system is based on an inverted microscope. A p-polarized incident light is directed onto the ITO surface via a high-numeric aperture objective with an incident angle slightly lower than the total internal reflection angle. The electric field is applied by a three-electrode electrochemical system, where the WE, RE, and CE represent working electrode, reference electrode, and counter electrode, respectively.
Materials and Reagents
ITO coated cover slips, 22 × 22 mm, thickness #1, 70-100 Ohms resistivity (SPI Supplies, catalog number: 06470-AB )
Silver wire (Alfa Aesar, catalog number: 11468-G9 )
Platinum wire (Alfa Aesar, catalog number: 10958-CB )
Reusable silicone well (SARSTEDT, flexiPERM®, catalog number: 94.6032.039 )
Streptavidin (VWR, catalog number: 97062-810 )
(3-glycidyloxypropyl) trimethoxylsilane (Sigma-Aldrich, catalog number: 440167 )
Bovine serum albumin (Sigma-Aldrich, catalog number: 0 5470 )
Goat IgG (MW = 150 kDa) (Abcam, catalog number: ab76907 )
Rabbit anti-goat IgG (MW = 150 kDa) (Invitrogen, catalog number: A16138 )
Biotin-PEG10k-NHS (Nanocs, catalog number: PG2-BNNS-10k )
Polystyrene (PS) nanoparticles, diameters of 50, 100, 150 and 200 nm (Bangs Laboratories, catalog numbers: PS2002 , PS2004 , PS2006 , and PS2008 )
(Optional) Sodium azide purification kit (Nanopartz, catalog number: PPZ-KIT-10-MAG )
1× phosphate buffered saline (Corning, catalog number: 21-040-CV )
Immersion oil type A (Cargille, catalog number: 16482 )
Isopropanol (Sigma-Aldrich, catalog number: 109827 )
Acetone (VWR, BDH®, BDH2002 )
Ethanol (Koptec’s Pure Ethanol 200 Proof, Decon Labs, catalog number: V1001 )
Ammonium Hydroxide (Mallinckrodt Chemicals, catalog number: 3256 )
Hydrogen peroxide (VWR, catalog number: BDH7814-3 )
H2O2/NH3·H2O/H2O (1:3:5) mixture (see Recipes)
0.1 mg/ml BSA blocking solution (see Recipes)
100 times diluted PBS buffer (see Recipes)
Equipment
Inverted optical microscope (Olympus, model: IX-81 )
60× NA 1.49 oil immersion TIRF objective (Olympus, model: APON60XOTIRF )
Antivibration optical table (Newport, model: RS2000 )
XYZ translation stage (Thorlabs, model: PT3 )
Superluminescent light emitting diode (SLED) (Superlum, model: SLD-260-HP-TOW-PD-670 )
SLED current driver (Superlum, model: PILOT4-AC )
CMOS camera (Hamamatsu, model: ORCA-Flash 4.0 )
Function generator (Agilent, model: 33521A )
Potentiostat (Pine Instrument Company, model: AFCBP1 )
USB data acquisition card (National Instruments, model: NI USB-6251 )
Vacuum pump (TOPSFLO, model: TM30A-B6-P9504 (V6004 ))
Plastic tubing, 0.02” ID × 0.06” OD (Saint-Gobain, TYGON®, model: AAD04103 )
Syringe with Luer-LokTM tip, 20 ml (Fisher Scientific, catalog number: 22-124-967 )
Perfusion manifold, 4 to 1 ports (Warner Instruments, model: MM-4 )
Syringe 1-way stopcock (B. Braun Medical Inc., catalog number: 455980 )
Dispensing needles (Weller, catalog number: KDS2312P )
Cover glass staining jars (Fisher Scientific, catalog number: 02-912-636 )
Ultrasonic cleaner (Fisher Scientific, model: FS20D )
Tweezers (Electron Microscopy Sciences, catalog number: 72750-D )
Computer (Processor: Intel Xeon E-2274G; RAM: 64 GB; Hard drive: Samsung SSD 970 PRO 1TB)
Software
HCImage Live (Hamamatsu)
MATLAB (MathWorks)
Fiji (https://imagej.net/Fiji)
Origin 2019 (OriginLab)
Office 365 (Microsoft)
Procedure
文章信息
版权信息
© 2021 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Ma, G., Wan, Z. and Wang, S. (2021). Simultaneous Imaging of Single Protein Size, Charge, and Binding Using A Protein Oscillation Approach. Bio-protocol 11(5): e3934. DOI: 10.21769/BioProtoc.3934.
分类
生物化学 > 蛋白质 > 成像
生物物理学 > 单分子技术
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link