- EN - English
- CN - 中文
Assay for Assessing Mucin Binding to Bacteria and Bacterial Proteins
评估粘蛋白与细菌和细菌蛋白结合的实验
(*contributed equally to this work) 发布: 2021年03月05日第11卷第5期 DOI: 10.21769/BioProtoc.3933 浏览次数: 4751
评审: Emilia KrypotouElizabeth LibbyAnonymous reviewer(s)

相关实验方案
从大肠杆菌中大规模纯化III型毒素-抗毒素核糖蛋白复合物及其成分,用于生物物理学研究
Parthasarathy Manikandan [...] Mahavir Singh
2023年07月05日 2160 阅读
Abstract
Legionella pneumophila, a Gram-negative bacterium and the causative agent of Legionnaires’ disease, exports over 300 effector proteins/virulence factors, through its type II (T2SS) and type IV secretion systems (T4SS). One such T2SS virulence factor, ChiA, not only functions as a chitinase, but also as a novel mucinase, which we believe aids ChiA-dependent virulence during lung infection. Previously published protocols manipulated wild-type L. pneumophila strain 130b and its chiA mutant to express plasmid-encoded GFP. Similarly, earlier studies demonstrated that wheat germ agglutinin (WGA) can be fluorescently labeled and can bind to mucins. In the current protocol, GFP-labeled bacteria were incubated with type II and type III porcine stomach mucins, which were then labeled with TexasRed-tagged WGA and analyzed by flow-cytometry to measure the binding of bacteria to mucins in the presence or absence of endogenous ChiA. In addition, we analysed binding of purified ChiA to type II and type III porcine stomach mucins. This protocol couples both bacterial and direct protein binding to mucins and is the first to measure Gram-negative bacterial binding to mucins using WGA and flow-cytometric analysis.
Graphic abstract:
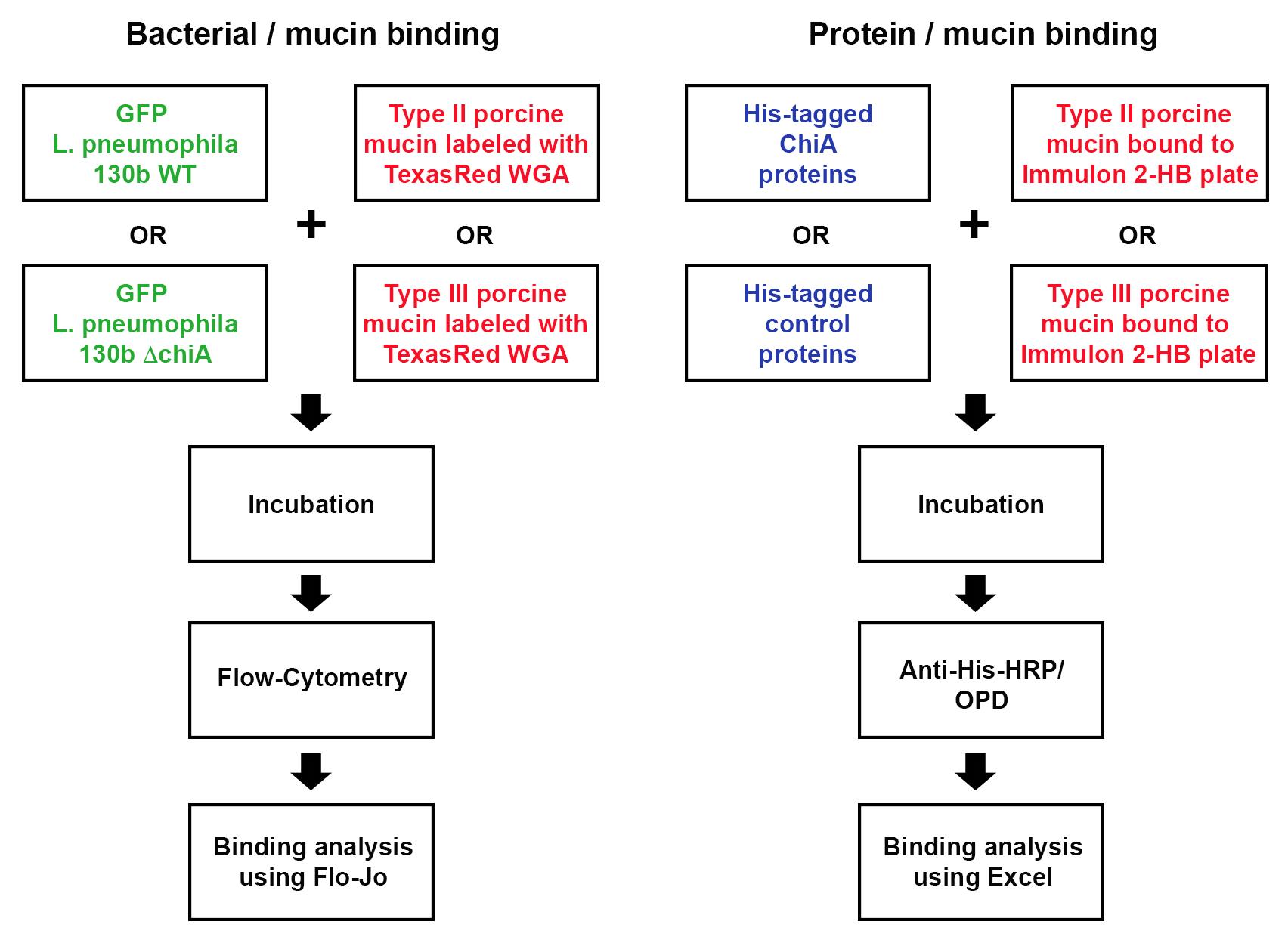
Strategy for assessing bacterial and protein binding to mucins
Background
Legionella pneumophila (Lpn), a Gram-negative bacterium, is the causative agent of Legionnaires’ disease, a severe form of pneumonia. Lpn is an intracellular pathogen that produces over 300 protein effectors that it secretes through either a type II secretion system (T2SS), or through a type IV secretion system (T4SS) (Hubber and Roy, 2010; White and Cianciotto, 2019). ChiA is one such T2SS protein effector. ChiA is an 81-kDa endochitinase that has a role in Lpn virulence during lung infection (Rehman et al., 2020). Lpn carrying a deletion of the ChiA gene (ΔchiA) shows decreased survival in the lungs of mice, compared to WT Lpn (DebRoy et al., 2006). While humans do not produce chitin, they do produce analogous glycoproteins, mucins, that have known properties in interacting with and blocking infection of other pathogens. In our study, we showed, for the first time, that ChiA is able to both bind to and degrade mucins (Rehman et al., 2020). To determine if live bacteria were able to bind to mucins, we utilized our previously published protocol to manipulate wild-type Lpn strain 130b and its chiA mutant to express a GFP-producing plasmid (DebRoy et al., 2006; Rondelet and Condemine, 2013). Furthermore, earlier studies showed that wheat germ agglutinin (WGA) binds to mucins (Bhavanandan and Katlic, 1979; Valdizan et al., 1992). Therefore, we utilized porcine stomach mucins which we labeled with TexasRed-conjugated WGA (Model et al., 2009). To determine whether ChiA directly binds to these mucins, we also used an ELISA based-assay with purified recombinant (N-terminally His-tagged) ChiA and detected binding using anti-His6 antibodies.
Although mucin binding to bacteria has been studied previously (Naughton et al., 2014), and fluorescently labeled WGA has been studied in the context of Gram-positive bacteria (Fife et al., 2000) this is the first protocol to directly label mucins and bacteria and then utilize flow cytometry to measure mucin binding to a Gram-negative bacterium. Furthermore, by analyzing both bacterial binding to mucins and the binding of purified proteins to mucins, this protocol provides insight into synergistic binding of different surface exposed bacterial proteins to mucins. This protocol can be further applied to study mucin binding to other Gram-negative bacteria.
Part I: Bacterial Mucin Binding with Flow Cytometry
Materials and Reagents
Legionella pneumophila Brenner et al. (1979) (ATCC® BAA-74TM)
WT 130b purchased from ATCC (see above)
ChiA mutant as previously reported (DebRoy et al., 2006)
GFP plasmid (pMGFP), as previously reported, was derived from pMMB-GRN/pMMB-Gent (addgene 45475). GFP is expressed from a Ptac promotor and therefore IPTG is required (White and Cianciotto, 2016) (Sturgill-Koszycki and Swanson, 2000). While copy number in Legionella is unknown, the PMMB-Gent derives from PMMB67EH (ATCC 37622)
1× Phosphate Buffered Saline (PBS) (Corning, catalog number: 21-040-CM )
IPTG (Sigma-Aldrich, catalog number: I6758 )
Type II porcine stomach mucin (Sigma-Aldrich, catalog number: M1778 )
Type III porcine stomach mucin (Sigma-Aldrich, catalog number: M2378 )
TexasRed-tagged WGA (ThermoFisher Scientific, catalog number: W21405 )
Sodium carbonate, Na2CO3 (Sigma-Aldrich, catalog number: S7795 )
Sodium bicarbonate, NaHCO3 (Sigma-Aldrich, catalog number: S5761 )
Buffered Charcoal Yeast Extract (BCYE) agar plates (see Recipes)
ACES (Sigma-Aldrich, catalog number: A9758 )
KOH (Sigma-Aldrich, catalog number: 221473 )
Yeast Extract (Sigma-Aldrich, catalog number: Y1625 )
α-Ketoglutaric acid sodium salt (Sigma-Aldrich, catalog number: K1875 )
Activated Charcoal (Sigma-Aldrich, catalog number: C9157 )
Bacteriological Agar (Sigma-Aldrich, catalog number: A5306 )
L-cysteine HCl (Sigma-Aldrich, catalog number: C1276 )
Ferric pyrophosphate (Sigma-Aldrich, catalog number: P6526 )
50 mM Carb/Bicarb Buffer (see Recipes)
Mucin solution (see Recipes)
Equipment
Flow Cytometer (https://www.bdbiosciences.com/en-us/go-campaign/lsr-ii-comp-cont) using Blue Laser (488 nm), Long pass Filter 600 and 505, Band Pass Filter 600-620 and 500-550 (BD Biosciences, model: LSR II )
General Purpose UV/Vis Spectrophotometer (Beckman Coulter, model: DU720 )
Forced Air Microbiological Incubators (VWR, catalog number: 89511-430 )
Software
GraphPad Prism version 8.0.0 for Mac (GraphPad Software, San Diego, California USA, www.graphpad.com)
FlowJoTM Software for Mac, Version 8.8.6. (Ashland, OR: Becton, Dickinson and Company, www.flowjo.com)
Procedure
文章信息
版权信息
© 2021 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Grigoryeva, L. S., Rehman, S., White, R. C., Garnett, J. A. and Cianciotto, N. P. (2021). Assay for Assessing Mucin Binding to Bacteria and Bacterial Proteins. Bio-protocol 11(5): e3933. DOI: 10.21769/BioProtoc.3933.
分类
微生物学 > 微生物-宿主相互作用 > 细菌
微生物学 > 微生物生物化学 > 蛋白质 > 相互作用
细胞生物学 > 基于细胞的分析方法 > 流式细胞术
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link