- EN - English
- CN - 中文
An Imaging Flow Cytometry Method to Measure Citrullination of H4 Histone as a Read-out for Neutrophil Extracellular Traps Formation
一种成像流式细胞术测定组蛋白H4瓜氨酸化的方法,作为中性粒细胞胞外陷阱形成的信号通路
(*contributed equally to this work) 发布: 2021年02月20日第11卷第4期 DOI: 10.21769/BioProtoc.3927 浏览次数: 5753
评审: Alessandro DidonnaFelix M. WensveenIngrid Lin
Abstract
The formation of neutrophil extracellular traps (NETs) is thought to play a critical role in infections and propagating sterile inflammation. Histone citrullination is an essential and early step in NETs formation, detectable prior to the formation of the hallmark extracellular DNA-scaffolded strands. In addition to the classical microscopy method, new technologies are being developed for studies of NETs and their detection, both for research and clinical purposes. Classical microscopy studies of NETs are subjective, low throughput and semi-quantitative, and limited in their ability to capture the early steps. We have developed this novel Imaging Flow Cytometry (IFC) method that specifically identifies and quantifies citrullination of histone H4 as a NETs marker and its relationship with other alterations at nuclear and cellular level. These include nuclear decondensation and super-condensation, multi-lobulated nuclei versus 1-lobe nuclei and cell membrane damage. NETs markers can be quantified following variable periods of treatment with NETs inducers, prior to the formation of the specific extracellular DNA-scaffolded strands. Because these high throughput image-based cell analysis features can be performed with statistical rigor, this protocol is suited for both experimental and clinical applications as well as clinical evaluations of NETosis as a biomarker.
Keywords: NETs detection (中性粒胞外菌网的探测)Background
Activated neutrophils are rapidly recruited to sites of infection and injury; they contribute to host defense against pathogens and inflammation. About two decades ago, it was observed for the first time that in response to pathogens, a fraction of the neutrophil population can undergo a distinct type of cellular death, different from apoptosis or necrosis. This involves early chromatin decondensation, co-localization of the nuclear and granular compartments and finally, release of this mix into the environment. The extracellular DNA-scaffolded strands anchoring nuclear components (such as histones) or cellular constituents, some with strong anti-pathogen properties like elastase or myeloperoxidase (MPO), are known as “neutrophil extracellular traps” or NETs and have effective antimicrobial activity (Brinkmann et al., 2004). More recent evidence shows that NETs also associate with non-infectious conditions including cancers, systemic lupus erythematosus, sickle cell disease, atherosclerosis, and thrombosis (Jorch and Kubes, 2017). It is yet not understood whether the NETs, or any of their components (cell-free DNA or histones) contribute to the pathology of these disorders as drivers, or whether they are by-products of an unbalanced immune response. NETs might also function as biomarkers in certain diseases and provide information regarding the efficacy of a treatment regimen (Barnado et al., 2016). NETs-associated components such as elastase or MPO are present in the plasma of individuals with autoimmune diseases and also in infections, which complicates the specificity of these molecules as biomarkers. In this light there is a great need for new methodologies for NETs characterization that offers consistent criteria between studies, and are reliable for both mechanistic and clinical applications.
Other NETs detection and quantification methods are being developed in addition to the classical widely used immunofluorescence microscopy technique. These include use of confocal microscopy that provides information on the structure of the NETs (Santos et al., 2018), ELISA-based assay to quantify the citrullinated histone 3 (H3cit) in human plasma (Thalin et al., 2017), high throughput live detection of NETosis- and apoptosis-related nuclear changes using membrane-permeable and -impermeable nuclear dyes in human neutrophils (Gupta et al., 2018), quantification of free chromatin (predominantly associated with elastase) in human whole blood by using microfluidics (Muldur et al., 2018). Several studies monitored NETs components (H3cit, MPO and extracellular DNA) in human and mice by using conventional flow cytometry (Gavillet et al., 2015; Masuda et al., 2017; Lee et al., 2018; Zharkova et al., 2019). While this technique allows high-throughput analysis it also does have noteworthy limitations, regarding the choice of the probes, the preparation of the samples and the way gating impacts final conclusions (Manda-Handzlik et al., 2016; Masuda et al., 2017). On the other hand, while Imaging Flow Cytometry (IFC) does allow high-throughput analysis, it also combines visualization and analysis features from both conventional flow cytometry and fluorescence microscopy (Basiji, 2016). NETs-associated morphological nuclear changes (i.e., chromatin decondensation and DNA trails) and whether and to what degree MPO co-localized with the nuclear compartment were investigated with IFC (Zhao et al., 2015; Ginley et al., 2017; Pelletier et al., 2017). Thus, different markers and different methods are currently employed to detect and quantify NETs in vitro. PAD4-mediated histone citrullination has been long designated as a hallmark of NETs formation, and thus a desirable marker to follow. More recent reports, however, predominantly in the mouse model, suggest that PAD4-mediated citrullination of histone 3 (H3cit) is stimulus-dependent (Neeli and Radic, 2013; Claushuis et al., 2018; Guiducci et al., 2018; Liang et al., 2018) and therefore the utility of this site as a NETs marker might be limited.
Here, we describe in detail a novel IFC protocol that allows specific detection and quantification of citrullination of histone 4 (H4cit3) as a NETs marker in whole neutrophils, prior to the release of the DNA and the cytoplasmic protein strands into the extracellular space. Other analysis parameters that look at nuclear and cellular morphological changes (nuclear decondensation and supercondensation, multi-lobulated nuclei and cell membrane damage) can bring additional information on the behavior of the analyzed neutrophil population. To establish our methodology we determined the responses of healthy human neutrophils treated for 5 different periods of time (between 2 min and 60 min) with NETs agonists: pharmacological inducers, PMA and calcium ionophore (a well-known inducer of histone citrullination, and for our experimental conditions, the positive control), Hemin (an inducer present under hemolytic conditions) and LPS and IL-8 (inducers associated with infectious pathogens). We used in vitro fluorescence microscopy to confirm the formation of DNA-elastase-MPO strands in healthy neutrophils treated with the stimuli used for the IFC tests (Barbu et al., 2020). We further validated this technique in untreated and Hemin-treated neutrophils from healthy donors and patients with sickle cell disease at steady state (Barbu et al., 2019).
The increased sensitivity and objective analysis make this methodology highly suitable for NETs detection in both research and clinical studies, that explore mechanistic answers or possible therapeutic strategies.
This protocol has 4 major parts: one-step neutrophil purification, induction of NETosis, specific staining, IFC acquisition and analysis (as highlighted in Figures 1A and 1C), with the first 3 steps requiring 3 days and up to one week to complete all steps.
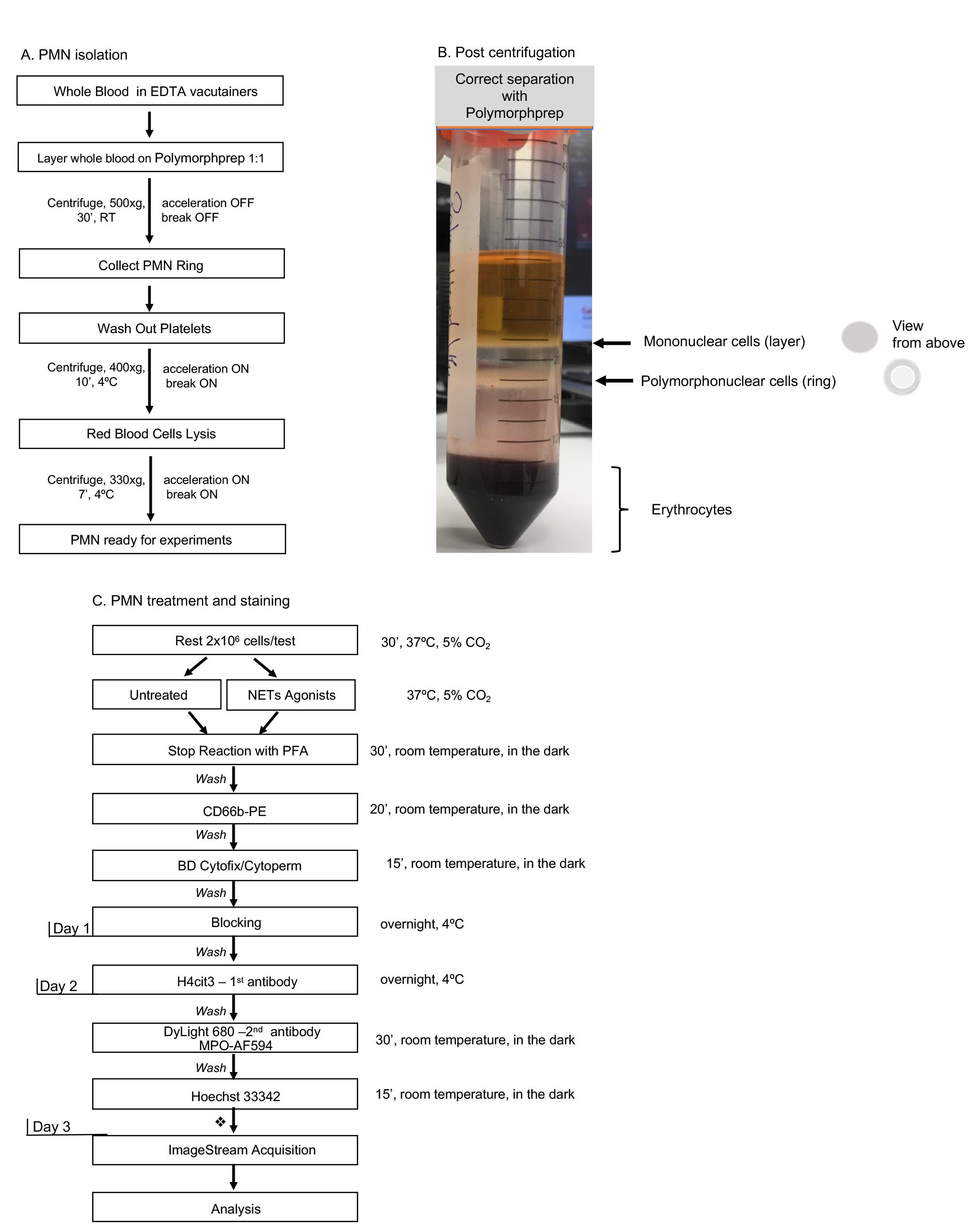
Figure 1. Day-by-day description of the major steps for specific detection of NETosis markers by Imaging Flow Cytometry. A. One-step polymorphoponuclear (PMN) cells isolation from whole blood with Polymorphprep medium; with this method neutrophils purity should be consistently above 80%. Total processing time: 90 min. B. An example of correct separation of whole blood by using Polymorphprep gradient medium. C. Detailed description of polymorphonuclear cells treatment and staining procedure. A time recommendation is not included for the neutrophil stimulation step as researchers should choose an appropriate treatment time according to their experimental purpose. Each washing step might need up to 20 min to complete, depending on the number of samples processed. This time should be taken into account while calculating total time required to complete all steps per day. Short final wash followed by samples storage at 4 °C. Total stimulation and staining processing time for Day 1: 4+ h.
Materials and Reagents
See Through, Lavender, 10.0 V, 16 × 100 mm, Plastic Tube with BD Hemograd Closure (BD, catalog number: BD366643 )
Polypropylene Conical Tubes, 50 ml (Fisher Scientific, Corning, catalog number: 07-203-510 )
Centrifuge tubes, 5 ml, conical bottom, sterile (Benchmark Scientific, catalog number: C1005-T5-ST )
Centrifuge tubes 1.5 ml, mixed neon colors (USA Scientific, catalog number: 1415-1448 )
Rapid flow sterile disposable filter units with PES membrane, capacity 500 ml, pore size 0.45 μm (Thermo Scientific Nalgene, catalog number: 09-740-63E )
Aluminum foil
Polymorphprep gradient medium (Cosmo Bio USA, catalog number: AXS-1114683 )
Note: Polymorphprep should be stored at room temperature, in the dark. Long term exposure to light affects its efficacy.
RPMI-1640 without L-glutamine (Lonza, catalog number: 12-167F )
L-glutamine 200 mM (Thermo Fisher, catalog number: 25030-081 )
Note: Aliquot in one-time use fractions and store at -80 °C. Thaw in water bath at 37 °C to dissolve the white sediment, then add to the RPMI to make the complete neutrophil medium.
Stock 32% Paraformaldehyde (PFA) Aqueous Solution, EM Grade (Science Services, catalog number: E15714 ). Once open use within days
Ultrapure 0.5 M EDTA pH 8.0 (Thermo Fisher, Invitrogen, catalog number: 15575020 )
DPBS 1×, no calcium chloride, no magnesium chloride (Gibco, catalog number: 141190-136 )
Cell Culture Grade Water (Corning, catalog number: 25-055-CV )
Bovine Serum Albumin, heat shock fraction, protease free, pH 7.0, ≥98% (Millipore Sigma, catalog number A3294 )
Potassium Chloride Granular (Mallinckrodt, catalog number: 6858 )
Dimethyl Sulfoxide (DMSO), ≥99.5% (GC), plant cell culture tested (Sigma-Aldrich, catalog number: D4540 )
Hemin from bovine, ≥90% (Millipore Sigma, catalog number: 9039 )
Note: Prepare a 40 mM stock in DMSO, aliquot in single use fractions and store at at -20 °C. Mix well when preparing the working solutions. Add a DMSO-only control with the tested samples.
PMA, for use in molecular biology applications, ≥99%, HPLC (Millipore Sigma, catalog number: P1585 )
Note: Prepare a stock solution in DMSO and freeze single use aliquots at -20 °C.
LPS-EB, Ultrapure, E. coli 0111:B4 (InvivoGen, catalog number: tlrl-3pelps )
Note: Prepare a stock solution in ddH2O, aliquot and freeze at -20 °C. Limit the thaw-freeze cycles to three.
Recombinant Human IL-8 (CXCL7, 77aa) (Peprotech, catalog number: 200-08 )
Calcium Ionophore A23187 ≥98% (TLC), powder (Millipore Sigma, catalog number: C7522 )
Anti-Human CD66b-PE, clone G10F5 (Biolegend, catalog number: 305106 )
Rabbit polyclonal anti-histone H4, citrulline 3 (H4cit3, Millipore Sigma, catalog number: 07-596 ) (see Notes)
Goat anti-Rabbit IgG (H+L) Secondary Antibody, DyLight 680 (Thermo Fisher, catalog number: 35568 ) (see Notes)
MPO Polyclonal Antibody, AlexaFluor 594 conjugated (Bioss Antibodies, catalog number: bs-4943R-A594 )
Hoechst 33342 (BD Pharmingen, catalog number: 561908 )
Gelatin from Porcine Skin, powder, Type A, suitable for cell culture (Millipore Sigma, catalog number: G1890 )
Note: Prepare 2% stock and keep it at 4 °C for up to 6 months. Open in sterile hood only. Monitor for signs of bacterial or fungal contamination.
BD Cytofix/Cytoperm kit (BD Biosciences, catalog number: 554722 ). Use only the Cytofix/Cytoperm solution in this protocol
Wash Buffer (see Recipes)
Blocking buffer (see Recipes)
Paraformaldehyde 8% working solution (see Recipes)
Porcine skin gelatin stock 2% (see Recipes)
0.6 M KCl (see Recipes)
Neutrophils complete medium (see Recipes)
Equipment
Water bath at 37 °C
LabGard® ES, Class II, Type A2, Biological Safety Cabinet (NuAire, Plymouth, MN)
Benchtop Centrifuge with swinging bucket rotor (Beckman Coulter, model: Allegra X-14R )
Centrifuge (Thermo Fisher Scientific, model: Sorvall ST 16R )
CO2 Incubator (Thermo Fisher Scientific, model: Heracell VIOS 160i )
Amnis ImageStream Mark II imaging flow cytometer (Luminex Corporation, Austin, TX, USA)
Software
Amnis INSPIRE (Luminex Corporation, Austin, TX, USA) for data acquisition
Amnis IDEAS (Luminex Corporation, Austin, TX, USA) for data analysis, available for download on the company’s website
Procedure
文章信息
版权信息
© 2021 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Barbu, E. A., Dominical, V. M., Mendelsohn, L. and Thein, S. L. (2021). An Imaging Flow Cytometry Method to Measure Citrullination of H4 Histone as a Read-out for Neutrophil Extracellular Traps Formation. Bio-protocol 11(4): e3927. DOI: 10.21769/BioProtoc.3927.
分类
免疫学 > 炎症性疾病 > 炎性小体
微生物学 > 微生物细胞生物学 > 细胞染色
细胞生物学 > 细胞成像
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link













