- EN - English
- CN - 中文
Bioorthogonal Labeling and Chemoselective Functionalization of Lung Extracellular Matrix
肺细胞外基质的生物正交标记和化学选择性功能化
发布: 2021年02月20日第11卷第4期 DOI: 10.21769/BioProtoc.3922 浏览次数: 4401
评审: Scott LaughlinWilliam C. W. Chen
Abstract
Decellularized extracellular matrix (ECM) biomaterials derived from native tissues and organs are widely used for tissue engineering and wound repair. To boost their regenerative potential, ECM biomaterials can be functionalized via the immobilization of bioactive molecules. To enable ECM functionalization in a chemoselective manner, we have recently reported an effective approach for labeling native organ ECM with the click chemistry-reactive azide ligand via physiologic post-translational glycosylation. Here, using the rat lung as a model, we provide a detailed protocol for in vivo and ex vivo metabolic azide labeling of the native organ ECM using N-Azidoacetylgalactosamine-tetraacylated (Ac4GalNAz), together with procedures for decellularization and labeling characterization. Our approach enables specific and robust ECM labeling within three days in vivo or within one day during ex vivo organ culture. The resulting ECM labeling remains stable following decellularization. With our approach, ECM biomaterials can be functionalized with desired alkyne-modified biomolecules, such as growth factors and glycosaminoglycans, for tissue engineering and regenerative applications.
Keywords: Extracellular matrix (细胞外基质)Background
The extracellular matrix (ECM) is a hydrated network scaffold composed of non-cellular components from a given tissue or organ, and plays key roles in supporting the activities of residential cells through its contained bioactive elements, such as fibrous proteins, growth factors and glycosaminoglycans (GAGs) (Theocharis et al., 2016). Decellularized ECM materials have been widely used for tissue engineering due to their inherent biocompatibility, highly preserved tissue architecture and biomechanical properties (Ott et al., 2008 and 2010; Petersen et al., 2010; Wagner et al., 2014). Despite the importance, one critical barrier for applying decellularized ECM to regenerative applications is that the harsh decellularization conditions usually cause dramatic loss or denaturation of key bioactive components embedded within the ECM (Reing et al., 2010; Uhl et al., 2020). To overcome this challenge, emerging efforts are underway to functionalize ECM materials via the immobilization of desired bioactive molecules, such as growth factors and GAGs, to facilitate tissue repair and regeneration.
For ECM biomaterial functionalization, amine-reactive chemistry has been widely used, due to the wide availability of amines in almost all ECM proteins. However, such high abundance of amine ligands within the ECM leads to limited control over reaction specificity and poses potential risk of compromising the desired biochemical and biomechanical properties of ECM biomaterials (Wissink et al., 2001; Chiu and Radisic, 2010; Grover et al., 2012; Davidenko et al., 2015). Chemoselective engineering of ECM materials, via the specific conjugation between two ligands that do not exist in native biological systems, offers a promising solution to this challenge. The azide-to-alkyne click conjugation is one such chemistry that has been widely used for protein engineering and has demonstrated excellent biocompatibility for in vivo and in vitro applications (Laughlin and Bertozzi, 2007; Chang et al., 2010; Sletten and Bertozzi, 2011).
Here, we describe a metabolic labeling approach to covalently incorporate the azide ligand into organ ECM using an azido monosaccharide, azidoacetylgalactosamine-tetraacylated (Ac4GalNAz). Ac4GalNAz can be incorporated as post-translational glycan modification during new ECM protein synthesis both in vivo and during ex vivo organ culture. The resulting click-reactive azide ligands within the ECM remain stable following decellularization and enable further functionalization with desired biomolecules bearing the complementary alkyne ligand using the copper-catalyzed, azide-alkyne cycloaddition (Ren et al., 2018). The protocol described here, using the rat lung as a model, is expected to be applicable to label the ECM of a wide variety of organs, such as the heart, liver, kidney and blood vessel. We also expect our protocol to be applicable to engineer ECM biomaterials derived from other animal sources, such as the mouse and porcine models (Ren et al., 2018).
Materials and Reagents
27G × 1/2 tuberculin syringe (BD, catalog number: 305620 )
Reusable Stainless Steel Dispensing Needle with Luer Lock Connection, Blunt, 18 Gauge (McMaster Carr, catalog number: 6710A44 )
Dremel rotary tool kit (Dremel, catalog number: 8220 )
Luer-Lok Syringe 1 ml (BD, catalog number: 309628 )
Luer-Lok Syringe 10 ml (BD, catalog number: 302995 )
Luer-Lok Syringe 30 ml (BD, catalog number: 302832 )
Petri Dish, PS, 145/20 mm (Greiner Bio-One, catalog number: 639102 )
2-0 PERMA-HAND Silk Suture (Ethicon, catalog number: LA55G )
Sterile Gauze Sponges (Covidien, catalog number: 2187 )
PVDF Transfer Membrane (Thermo Fisher Scientific, catalog number: 88518 ), storage: RT
Sprague Dawley rats (100-250 gram) (Charles River Laboratories, Strain Code: 400)
N-azidoacetylgalactosamine-tetraacylated (Ac4GalNAz) (Click Chemistry Tools, catalog number: 1086 ), storage: -20 °C
Dimethyl Sulfoxide (DMSO) (Fisher Scientific, catalog number: BP231-100 ), storage: room temperature (RT)
Dulbecco′s Phosphate Buffered Saline (DPBS), 1× without calcium and magnesium (Corning, catalog number: 21-031-CV ), storage: RT
Dulbecco′s Modification of Eagle′s Medium (DMEM) (Corning, catalog number: 10-013-CV ), storage: 2-8 °C
FetalClone I Serum (Cytiva, catalog number: SH30080.03 ), storage: -20 °C
Antibiotic-Antimycotic (100×) (Thermo Fisher Scientific, catalog number: 15240062 ), storage: -5 °C to -20 °C
Sodium dodecyl sulfate (SDS) (Sigma-Aldrich, catalog number: 74255 ), storage: 2-8 °C
Triton X-100 (Sigma-Aldrich, catalog number: T8787 ), storage: RT
Phosphate Buffered Saline (PBS), 20× (Boston BioProducts, catalog number: BM-220 ), storage: RT
Paraformaldehyde (Sigma-Aldrich, catalog number: P6148 ), storage: 2-8 °C
Click-iT Cell Reaction Buffer Kit (Invitrogen, catalog number: C10269 ), storage: 2-6 °C
Biotin-PEG4-Alkyne (Click Chemistry Tools, catalog number: TA105 ), storage: -20 °C
Bovine Serum Albumin (BSA) (Fisher Scientific, catalog number: BP9706100 ), storage: 2-8 °C
Rabbit Polyclonal Laminin Antibody (Novus, catalog number: NB300-144 ), storage: -20 °C
Streptavidin, Alexa Fluor 647 conjugate (Thermo Fisher Scientific, catalog number: S21374 ), storage: -5 °C to -30 °C and protect from light
Donkey anti-Rabbit IgG (H+L) Secondary Antibody, Alexa Fluor Plus 488 (Thermo Fisher Scientific, catalog number: A32790 ), storage: 4 °C and protect from light
DAPI Fluoromount-G (SouthernBiotech, catalog number: 0100-20 ), storage: RT
Urea, ultrapure, 99% (Alfa Aesar, catalog number: J65769 ), storage: RT
HEPES (1 M) (Thermo Fisher Scientific, catalog number: 15630-080 ), storage: 2-8 °C
Protease Inhibitor Cocktail (100×) (Thermo Fisher Scientific, catalog number: 87786 ), storage: 4 °C
BCA Protein Assay Kit (Thermo Fisher Scientific, catalog number: 23225 ), storage: RT
4-15% Mini-PROTEAN TGX Precast Protein Gels (Bio-Rad Laboratories, catalog number: 4561085 ), storage: 4 °C
Tris Buffered Saline-Tween (TBST, 10×, with 0.5% Tween-20, pH 7.4) (Boston BioProducts, catalog number: IBB-181 ), storage: RT
High Sensitivity Streptavidin-HRP (Thermo Fisher Scientific, catalog number: 21130 ), storage: 4 °C
SuperSignal West Pico PLUS Chemiluminescent Substrate (Thermo Fisher Scientific, catalog number: 34580 ), storage: RT
SYPRO Ruby Protein Gel Stain (Thermo Fisher Scientific, catalog number: S12000 ), storage: RT
Ac4GalNAz Injection Solution (see Recipes)
Control Injection Solution (see Recipes)
Ex vivo Culture Medium (see Recipes)
Decell Solution (see Recipes)
2× Decell Solution (see Recipes)
1× PBS (pH 7.4) (see Recipes)
Fixation Solution (see Recipes)
Biotin-PEG4-Alkyne Click Reaction Mixture (see Recipes)
Urea Extraction Buffer (see Recipes)
1× TBST (pH 7.4) (see Recipes)
Blocking Buffer (see Recipes)
HRP-Imaging Mixture (see Recipes)
Equipment
Iris Scissors, 11.5 cm, straight (World Precision Instruments, catalog number: 503708-12 )
Pulmonary artery (PA) cannula (Figure 1)
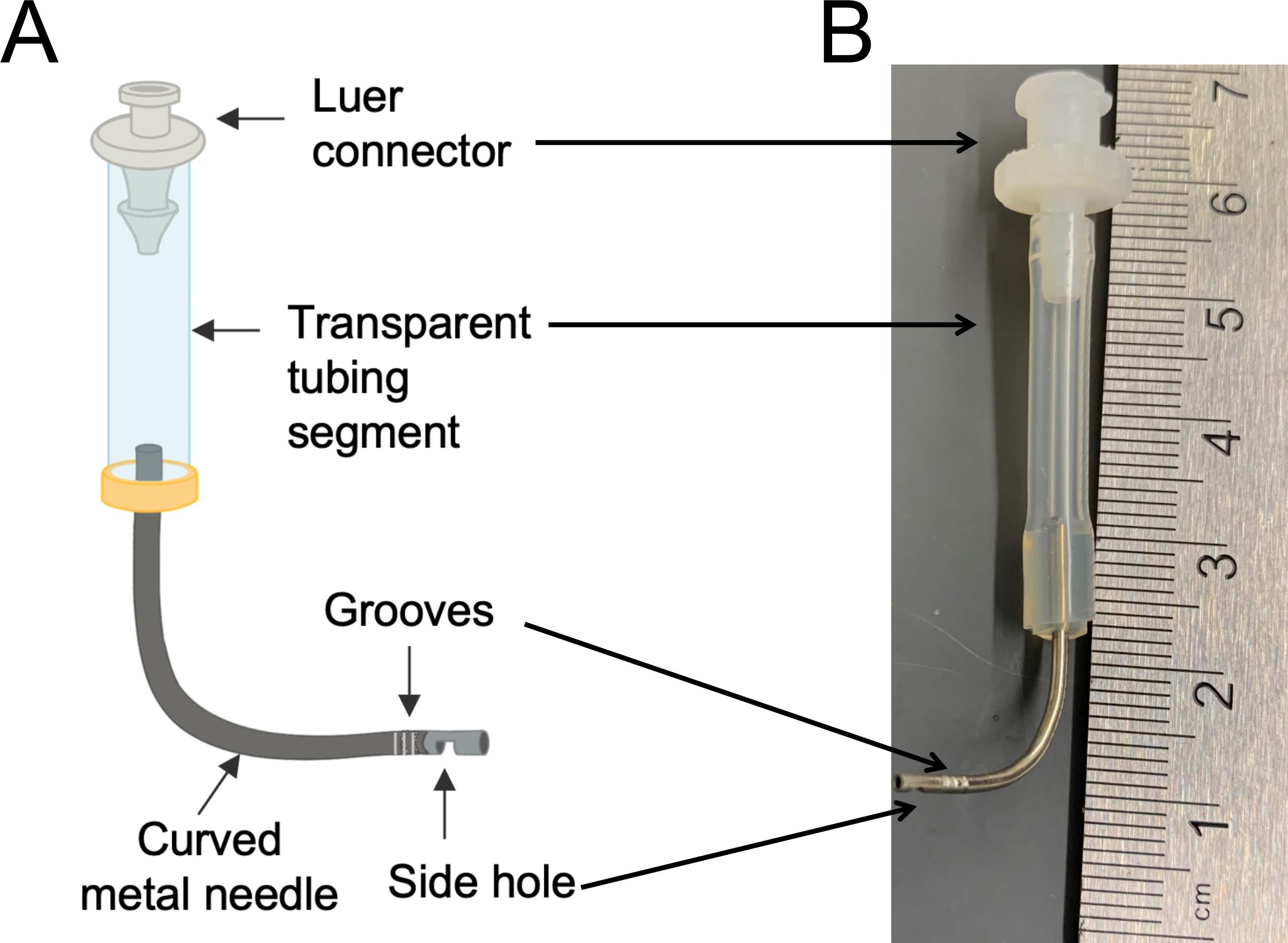
Figure 1. Custom-made PA cannula. A. Diagram. The cannula is composed of a top luer connector (1/8’, for connection with the bioreactor), a middle transparent tubing segment (1/8’, for detecting potential air bubbles), and a bottom curved metal needle (18 gauge, for connection with the PA). A small side hole at the end of the metal needle is generated using Dremel rotary tool with a sanding disc, to avoid potential perfusion blockage upon insertion into the PA. A series of grooves are created using a wire cutter around the tip of the metal needle to help stabilize the suture. B. An actual image of the cannula.Adson Forceps, straight, serrated (World Precision Instruments, catalog number: 503719-12 )
SILASTIC Thin-wall Silicone Laboratory Tubing (Dow Corning, catalog number: 508-009 )
Easy-Load Pump Head with 2-Channels for Precision Pump Tubing (Cole Parmer, catalog number: EW-77202-60 )
Masterflex L/S Digital Precision Modular Drive with Remote I/O and Benchtop Controller (Cole Parmer, catalog number: UX-07557-00 )
Autoclavable three-way valve (Cole Parmer, catalog number: EW-31200-80 )
Organ culture bioreactor (Figure 2)
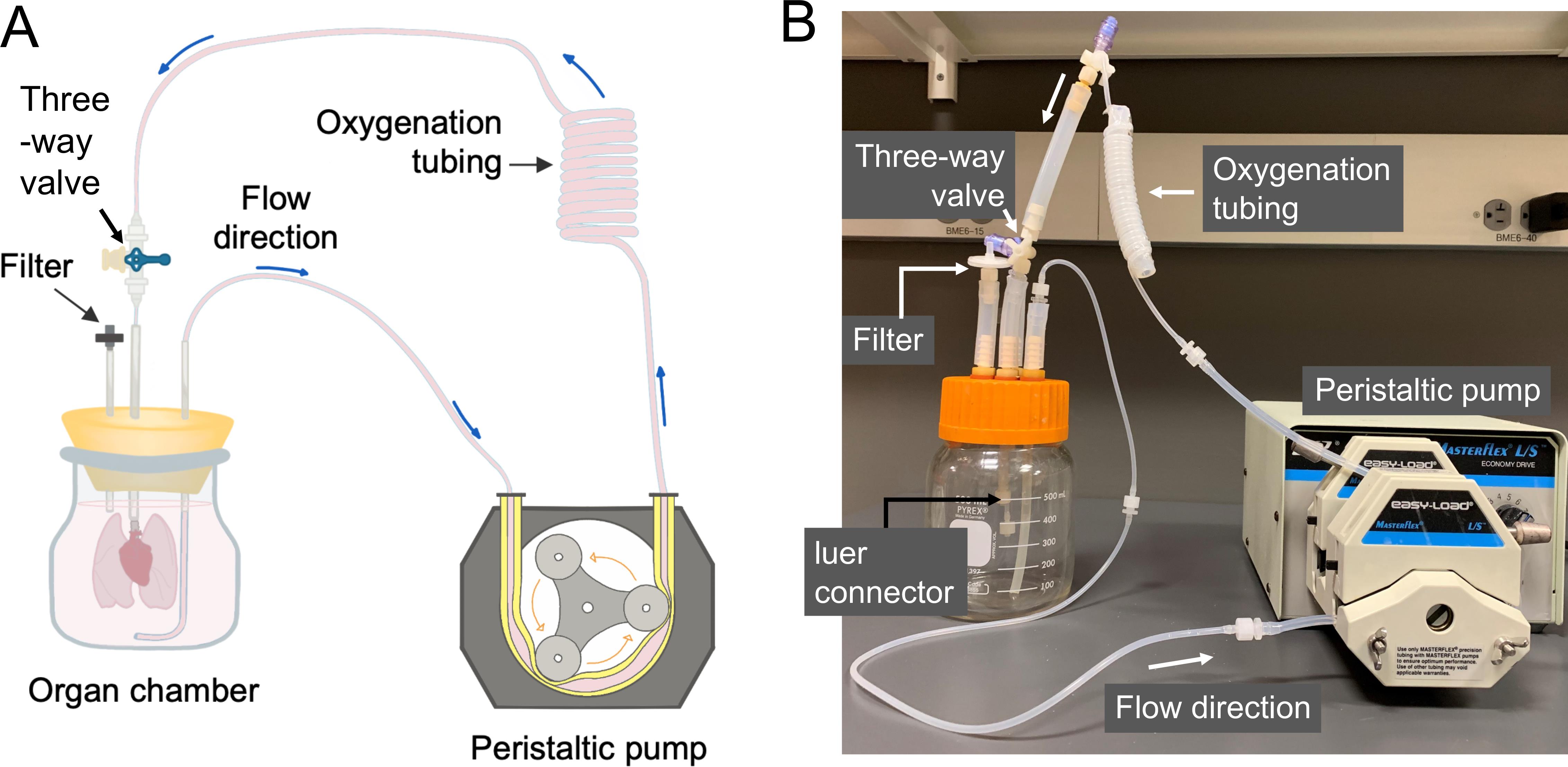
Figure 2. Organ culture bioreactor. A. Driven by a peristaltic pump, the culture medium is aspirated from the organ chamber, and perfuses through a series of thin-wall silicone tubing (for oxygenation) into the cannula leading to the PA of the lungs in culture. The filter on the organ chamber is for pressure equilibration. B. An actual image of the perfusion bioreactor.Decellularization chambers (Figure 3)
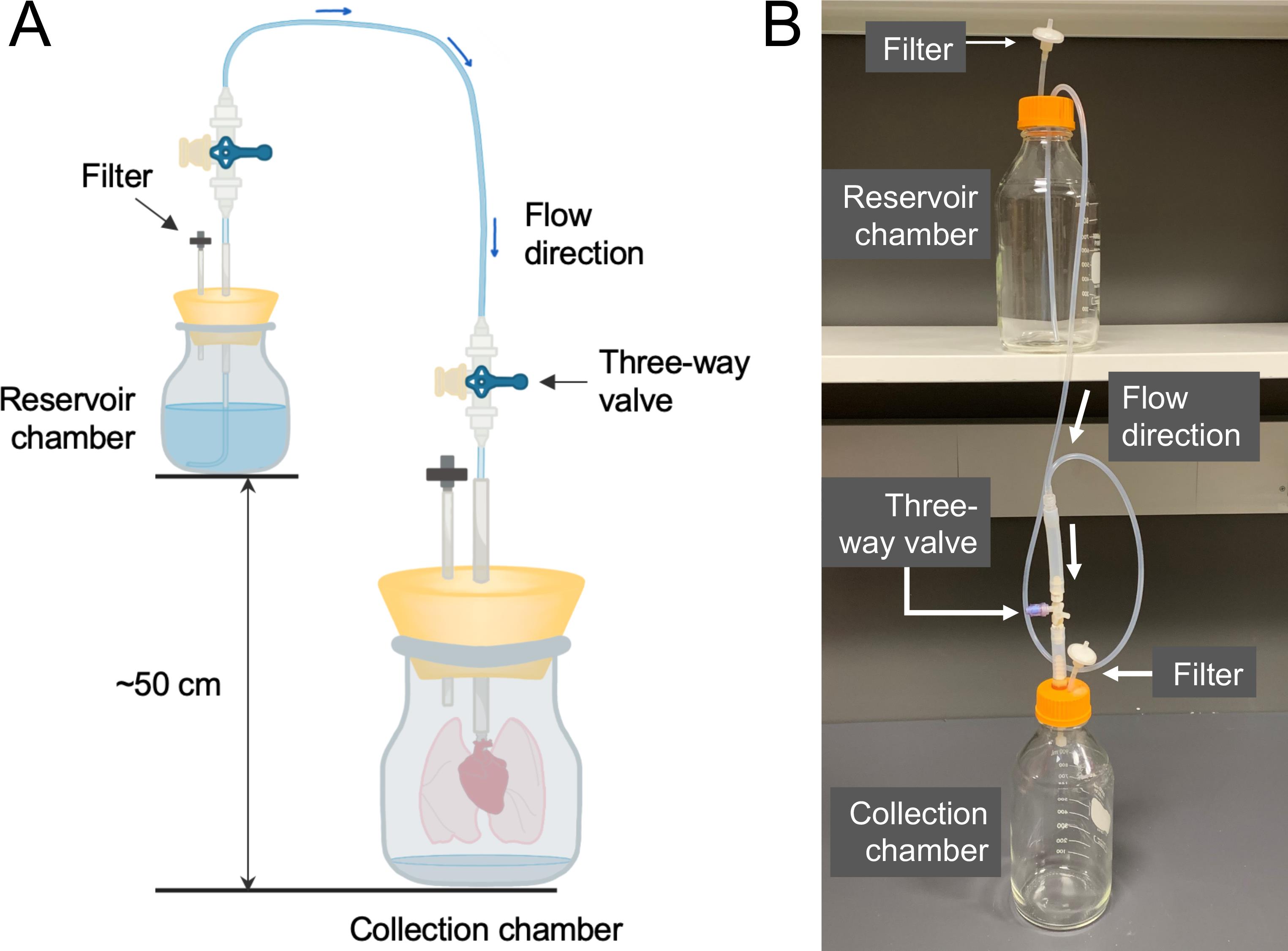
Figure 3. Decellularization chambers. A. There are two chambers in the system: the reservoir chamber containing fresh solutions for decellularization; and the collection chamber containing the lungs to be decellularized. The reservoir chamber should be placed approximately 50 cm higher than the collection chamber, which generates the gravity pressure to drive the fluid flow from the reservoir chamber into the lungs through the PA cannula. The filters on both chambers are for pressure equilibration. B. An actual image of the decellularization chambers.Semi-Automated Rotary Microtome (Leica Biosystems, catalog number: RM2245 )
EVOS FL Auto 2 Imaging System (Thermo Fisher Scientific, catalog number: AMAFD2000 )
Microcentrifuge (Eppendorf, catalog number: 5415R )
BeadBug Microtube Homogenizer (Benchmark Scientific, catalog number: D1030 )
Prefilled 2.0 ml Tubes, with Acid Washed Silica (Glass) Beads, 1.0 mm (Benchmark Scientific, catalog number: D1031-10 )
Mini-PROTEAN Tetra Vertical Electrophoresis Cell for Mini Precast Gels, with Mini Trans-Blot Module (Bio-Rad Laboratories, catalog number: 1658036 )
ChemiDoc Gel and Western blot imaging system (Bio-Rad Laboratories, catalog number: 12003153 )
Reach-in IR CO2 Incubator (Caron, catalog number: 7400-25-1 )
Procedure
文章信息
版权信息
© 2021 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Ling, Z., Xing, Y., Reinoso Jacome, E., Fok, S. W. and Ren, X. (2021). Bioorthogonal Labeling and Chemoselective Functionalization of Lung Extracellular Matrix. Bio-protocol 11(4): e3922. DOI: 10.21769/BioProtoc.3922.
分类
细胞生物学 > 组织分析
生物化学 > 糖类 > 糖蛋白
生物化学
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link













