- EN - English
- CN - 中文
In vitro Measurement of Membrane Attack Complex in RPE Cells
视网膜色素上皮细胞膜攻击复合物的体外测定
发布: 2021年02月20日第11卷第4期 DOI: 10.21769/BioProtoc.3916 浏览次数: 4489
评审: Lokesh KalekarMartin V KolevVemika Chandra

相关实验方案
高灵敏且可调控的 ATOM 荧光生物传感器:用于检测细胞中蛋白质靶点的亚细胞定位
Harsimranjit Sekhon [...] Stewart N. Loh
2025年03月20日 2240 阅读
Abstract
Initiation of the complement system results in the formation of a multiprotein pore termed the membrane attack complex (MAC, C5b-C9). MAC pores accumulate on a cell surface and can result in cell lysis. The retinal pigment epithelium (RPE) is a single monolayer of pigmented epithelial cells located at the posterior poll of the eye that forms the outer blood retinal barrier. RPE cells are highly polarized with apical microvilli and basolateral contact with Bruch’s membrane. In order to obtain biologically relevant polarized RPE cultures in vitro, RPE cells are seeded onto the apical side of a transwell filter and cultured for 4 weeks in low serum media. MAC formation on RPE cells has been reported to be sub-lytic. MAC formation can be achieved in vitro by introduction of normal human serum (NHS) to media following serum starvation for 24 h. NHS contains all serum complement proteins required to initiate complement activation and MAC formation. We combined in vitro RPE polarization and complement activation to visualize MAC formation in vitro utilizing confocal microscopy allowing for high resolution MAC imaging.
Keywords: Membrane attack complex (MAC) (攻膜复合体)Background
The Complement system is an evolutionary conserved innate immune pathway. There are three major independent yet overlapping pathways for complement activation that converge at the C3 convertase, the classical, the lectin and the alternative pathways. In the classical pathway, immune complexes (Antigen-Antibody complexes) bind C1 via the C1q subcomponent, then the C1s protease subunit cleaves complement factors C4 and C2. Fragments of these (C4bC2b) forms an enzyme complex ‘C3 convertase’, that cleaves C3 to C3b and releases the anaphylatoxin, C3a. The binding of C3b to the C3 convertase generates the C5 convertase (C4bC2bC3b). The lectin pathway is an analogous system, except that the initiating step is the binding by lectins to repetitive sugars on microbial surfaces. Mannose-associated serine proteases (MASPs) take the place of the C1 proteases. The alternative pathway (AP) continuously self-activates at a low level to generate C3b that deposits on pathogens or debris. C3b or C3(H2O) engages the rate limiting alternative pathway components, factors B (FB) and D (FD), to form the alternative C3 convertase (C3bBb), which in turn cleaves more C3 into C3a and C3b. The binding by another C3b to the C3 convertase generates the C5 convertase (C3bBbC3b). Properdin (P) is a positive regulator that stabilizes both the AP C3 and C5 convertases. The C5c convertase subsequently cleaves C5 to release the potent anaphylatoxin C5a, while C5b engages the terminal pathway and initiates the formation of the membrane attack complex (MAC). In this way activation of the complement system results in the assembly of transmembrane pores; MAC (Figure 1). MAC formation on gram-negative bacteria causes cell lysis. In nucleated cells MAC deposition can result in cell death by apoptosis (Nauta et al., 2002), direct lysis (Koski et al., 1983) or in some cases MAC can be sub-lytic and promote an inflammatory response (Niculescu and Rus, 2001). The number of MAC pores which form on a cells surface is directly related to cell lysis and as such MAC formation is tightly regulated by complement inhibitors. CD59 is a direct inhibitor of complement and is expressed on the cell surface of various host tissues (Meri et al., 1991). In addition to expression of complement inhibitors some nucleated cells can endocytose or exocytose MAC pores to prevent cell lysis (Morgan et al., 1987).
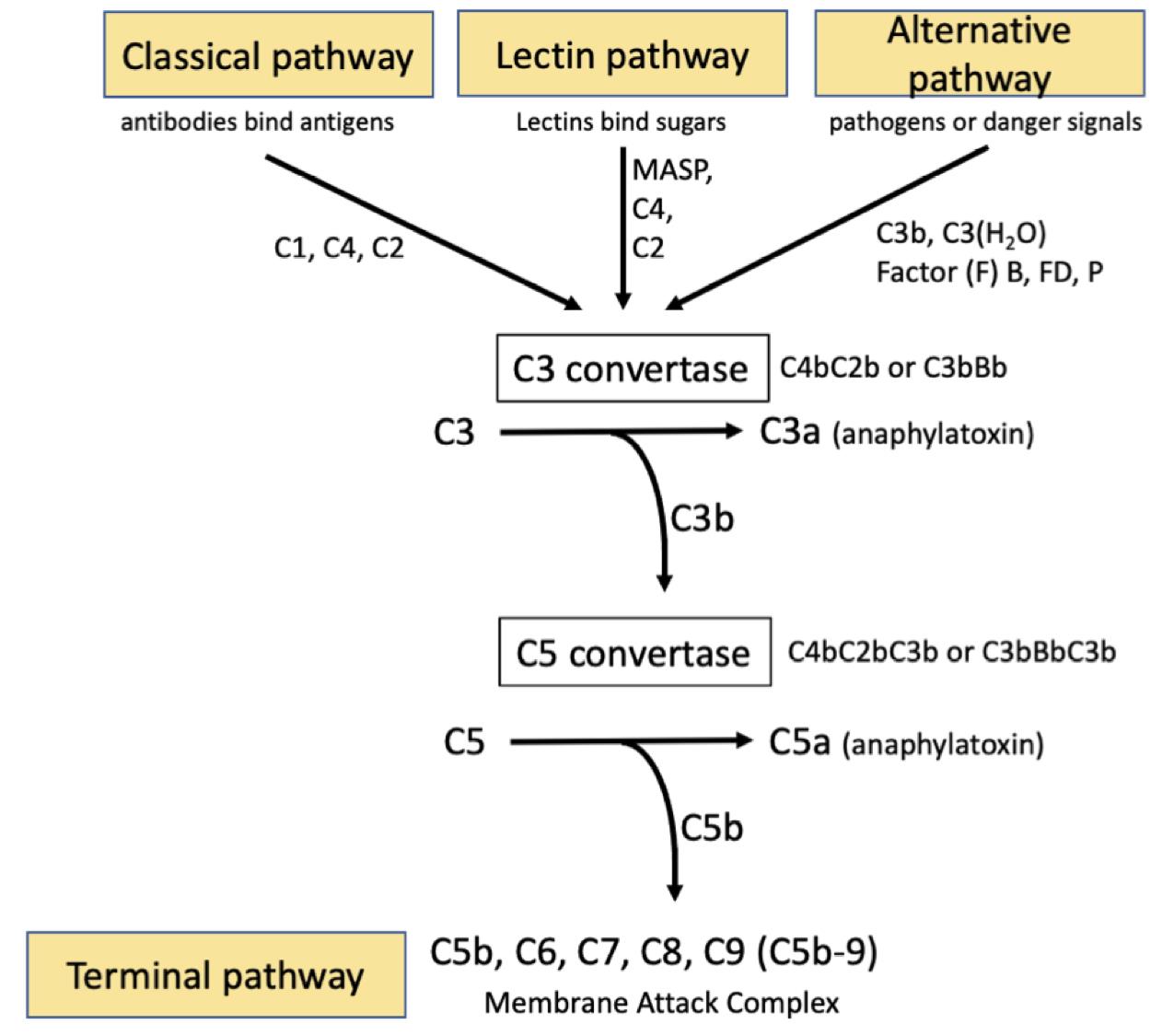
Figure 1. The three independent complement activating pathways leading to C3 convertase activation
Aberrant activation of complement and increased MAC formation is involved in the pathogenesis of age-related macular degeneration. MAC accumulation has been observed in donor eye tissue and is most abundant in the choroid in choriocapillaris endothelial cells (Mullins et al., 2014). MAC deposition is also observed although less readily on the retinal pigment epithelium (RPE). This may be explained by the finding that the RPE can rapidly endocytose and clear MAC pores (Georgiannakis et al., 2015). Sub-lytic MAC does not appear to be responsible for RPE cell death, however, it may act as a pro-inflammatory signaling mechanism contributing to overactivation of the innate immune system and leading ultimately to tissue degeneration (Mulfaul et al., 2020).
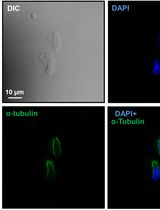
The RPE is a highly specialized monolayer of pigmented epithelial cells located between the neural retina and the choriocapillaris. In vivo, the RPE forms polarized monolayers with apical microvilli which interconnect with the overlying photoreceptor outer segments. The basolateral RPE has basal infoldings in close contact with Bruch’s membrane. The RPE is therefore an essential component of the outer blood retinal barrier and is necessary for maintenance of healthy vision. Monolayer polarization is essential to RPE function. In order to achieve a polarized monolayer of RPE cells in vitro, cells can be seeded on transwell filters and cultured for at least 4 weeks (Figure 2). This results in the formation of apical microvilli and polarized expression of tight junction proteins (Kannan et al., 2006; Sonoda et al., 2009). Polarizing RPE in vitro allows us to closer mimic RPE behavior in the mammalian eye.
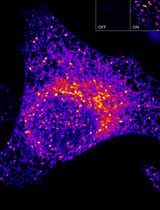
In order to study the contribution of MAC formation on the RPE in AMD we first polarized both the immortalized RPE cell line ARPE-19 or primary human fetal RPE (hfRPE). We then incubated cells with normal human serum (NHS) which contains all of the necessary components to drive complement activation and MAC deposition. NHS alone resulted in no visible MAC formation on ARPE-19 cells and only a small number of MAC were observed on hfRPE cells (Mulfaul et al., 2020). The apparent lack of MAC on RPE cells in spite of the presence of NHS is likely due to RPE expression of complement regulators including CD59 which directly inhibits MAC complex formation (Yang et al., 2009). Furthermore, if MAC complex’s do form the RPE can efficiently remove MAC by internalization of the MAC pore by endocytosis (Georgiannakis et al., 2015). We found that stimulation of RPE with an inflammatory insult prior to supplementation with NHS allowed MAC pores to persist on RPE cells that could be imaged by confocal microscopy. In a previous report, we demonstrated that the oxidative protein modification 2-(ω-carboxyethyl)pyrrole CEP, which is found in abundance in AMD donors (Gu et al., 2003) and is known to activate TLR2 and CD36, increased complement activation and MAC deposition on the RPE (Mulfaul et al., 2020). RPE cells activated with CEP in the presence of complement rich serum accumulate immunofluorescent measurable MAC pores. This protocol can be used as an in vitro model to study whether therapeutic targets can reduce MAC deposition on the RPE as we have previously demonstrated using an anti-TLR2 inhibitor (Mulfaul et al., 2020).
Materials and Reagents
T75 cell culture flasks (Corning, catalog number: CLS3275 )
15 ml conical tube (Corning, catalog number: CLS430791 )
0.4 μm polyester transwell inserts (VWR, catalog number: 734-1581 )
Polylysine coated slide (Thermo Fisher Scientific, catalog number: 10219280 ).
ARPE-19 cells (ATCC, catalog number: CRL-2302 )
Alexa Fluor 488 Goat Anti-Rabbit (Thermo Fisher Scientific, catalog number: A11034 )
Normal Goat Serum (Sigma-Aldrich, catalog number: G9023-10ml )
Collagen IV (Sigma-Aldrich, catalog number: C5533 )
DMEM/F-12 Ham (Sigma-Aldrich, catalog number: D8437-500ML )
Fetal Bovine Serum (Sigma-Aldrich, catalog number: F9665-500ML )
Pennicillin Streptomycin (Sigma-Aldrich, catalog number: P4333-100ML )
Phosphate Buffered Saline (Sigma-Aldrich, catalog number: D8662-500ML )
Trypsin-EDTA (Biosciences, catalog number: 25200-056 )
Normal human serum (Sigma-Aldrich, catalog number: H4522-20ml )
ZO-1 (Thermo Fisher Scientific, catalog number: 40-2200 )
Anti-C5b-9 (Santa Cruz Biotech, clone ae11, catalog number: sc58935 )
Hoechst (Sigma-Aldrich, catalog number: B2261-25MG )
Mowiol® 4-88 (Sigma-Aldrich, catalog number: 81381-50G )
Glycerol (Sigma-Aldrich, catalog number: G5516 )
Tris-HCl (Thermo Fisher Scientific, catalog number: 10724344 )
Paraformaldehyde (Sigma-Aldrich, catalog number: 158127 )
NaOH (Sigma-Aldrich, catalog number 567530 )
Triton X-100 (Sigma-Aldrich, catalog number: x100-500ML )
L-glutamine (Thermo Fisher Scientific, catalog number: 10378-016 )
Mowiol 4-88 (see Recipes)
4% PFA (see Recipes)
Equipment
Fume hood
Hemocytometer
-80 °C freezer
4 °C fridge
Tweezers
Biological Safety Cabinet Class ll
Heat Block
Confocal laser scanning microscope Axio Observer Z1 Inverted Microscope equipped with a Zeiss LSM 700 T-PMT Scanning unit and a 40x plan.
Procedure
文章信息
版权信息
© 2021 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Mulfaul, K. and Doyle, S. (2021). In vitro Measurement of Membrane Attack Complex in RPE Cells. Bio-protocol 11(4): e3916. DOI: 10.21769/BioProtoc.3916.
分类
免疫学 > 补体分析
神经科学 > 细胞机理
分子生物学 > 蛋白质 > 检测
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link