- EN - English
- CN - 中文
Efficient Transient Gene Knock-down in Tobacco Plants Using Carbon Nanocarriers
应用碳纳米载体对烟草基因进行高效瞬时敲减
发布: 2021年01月05日第11卷第1期 DOI: 10.21769/BioProtoc.3897 浏览次数: 6382
评审: Ayelign M. AdalHui Chen WuTohir A. Bozorov

相关实验方案

各种胁迫条件下根细胞DNA片段化和程序性细胞死亡的TUNEL法评估
Amit K. Tripathi [...] Sneh Lata Singla-Pareek
2017年08月20日 15037 阅读

使用Brick into the Gateway (BiG) 实验方法快速克隆细菌基因
Flaviani G. Pierdoná [...] Fabio T. S. Nogueira
2022年12月20日 2363 阅读
Abstract
Gene knock-down in plants is a useful approach to study genotype-phenotype relationships, render disease resistance to crops, and enable efficient biosynthesis of molecules in plants. Small interfering RNA (siRNA)-mediated gene silencing is one of the most common ways to achieve gene knock-down in plants. Traditionally, siRNA is delivered into intact plant cells by coding the siRNA sequences into DNA vectors, which are then delivered through viral and/or bacterial methods. In this protocol, we provide an alternative direct delivery method of siRNA molecules into intact plant cells for efficient transient gene knock-down in model tobacco plant, Nicotiana benthamiana, leaves. Our approach uses one dimensional carbon-based nanomaterials, single-walled carbon nanotubes (SWNTs), to deliver siRNA, and does not rely on viral/bacterial delivery. The distinct advantages of our method are i) there is no need for DNA coding of siRNA sequences, ii) this abiotic method could work in a broader range of plant species than biotic methods, and iii) there are fewer regulatory complications when using abiotic delivery methods, whereby gene silencing is transient without permanent modification of the plant genome.
Graphic abstract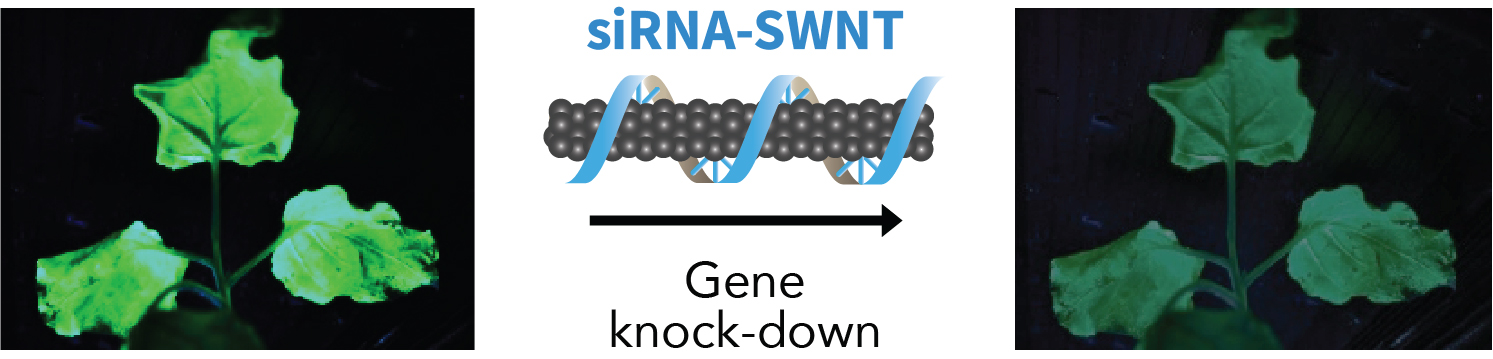
Background
Gene silencing through RNA interference (RNAi) was discovered in the early 1990s by plant researchers studying petunia flower coloring (Van der Krol et al., 1990). In RNAi, specifically in post-transcriptional gene silencing (PTGS), gene expression level is reduced through mRNA degradation caused by small RNA molecules – micro (miRNA) or small interfering (siRNA) RNA. RNAi has been a breakthrough technology, not only in plant research and biotechnology applications, but also for many other organisms, including human therapy applications (Sierakowska et al., 1996).
The first step of siRNA-mediated RNAi in plants is the delivery of siRNA molecules into plant cells. Delivery is a big bottleneck in plant biotechnology, given the presence of plant cell wall that acts as a physical barrier for the delivery of biotechnology-relevant cargoes such as DNA, RNA, and protein. In plants, siRNA delivery is most commonly accomplished through viral vector delivery via Agrobacterium tumefaciens. However, most plant viruses are limited in their host range (Silva et al., 2010) and the size of cargo they can efficiently deliver (Burch-Smith et al., 2004). Agrobacterium-mediated delivery is also limited in terms of plant host species, causes uncontrolled DNA integration into the plant nuclear genome, and results in constitutive expression of siRNA, which limits temporal control over gene silencing (Baltes et al., 2017).
Carbon nanotubes are one dimensional high-aspect-ratio nanomaterials that have many advantageous features for siRNA delivery in plants. First, given their needle-like structure with a small diameter (~1 nm), long length (~500 nm) and high stiffness, single-walled carbon nanotubes (SWNTs) have shown to transport across the plant cell wall and localize inside plant cells (Demirer et al., 2019b). Second, high surface area and diverse surface chemistry options of SWNTs enable delivery of diverse biological cargoes (Beyene et al., 2016; Del Bonis-O’Donnell et al., 2017; Demirer et al., 2020). Lastly, SWNTs have the ability to delay the intracellular degradation of biomolecular cargoes (Demirer et al., 2019a and 2020), which is especially valuable when working with fragile molecules like RNA.
Recently, we have developed a method to deliver siRNA molecules targeting the silencing of a transgenic GFP gene in Nicotiana benthamiana leaves, and an endogenous stress gene, ROQ1, using SWNTs (Demirer et al., 2020). In this approach, we first load sense and antisense strands of siRNA onto two separate SWNT nanoparticle solutions via pi-pi interactions that form between the sp2 carbon nanotube surface lattice and the aromatic bases of single stranded RNA (ssRNA). Next, we introduce an equimolar mixture of these RNA-SWNT solutions into intact plant leaves for GFP silencing. Our results demonstrate efficient silencing of GFP as assessed by confocal microscopy imaging, quantitative PCR (qPCR), and Western blotting, both for transgenic GFP and also for the endogenous ROQ1 gene, with disease-resistance applications (Demirer et al., 2020). This transient gene knock-down approach could be applied to other plant species, tissues, and target genes with minimal modifications. Additionally, the RNA loading method used in this study is not specific to siRNA, and thus, it can be adapted for the delivery of other types of nucleic acids with some optimization (e.g., guide RNA or messenger RNA for CRISPR genome editing applications).
Below, we provide a step-by-step protocol for the synthesis and characterization of siRNA loaded SWNTs, and the measurement of gene silencing efficiency in tobacco leaves through confocal imaging, qPCR and Western blotting (Figure 1).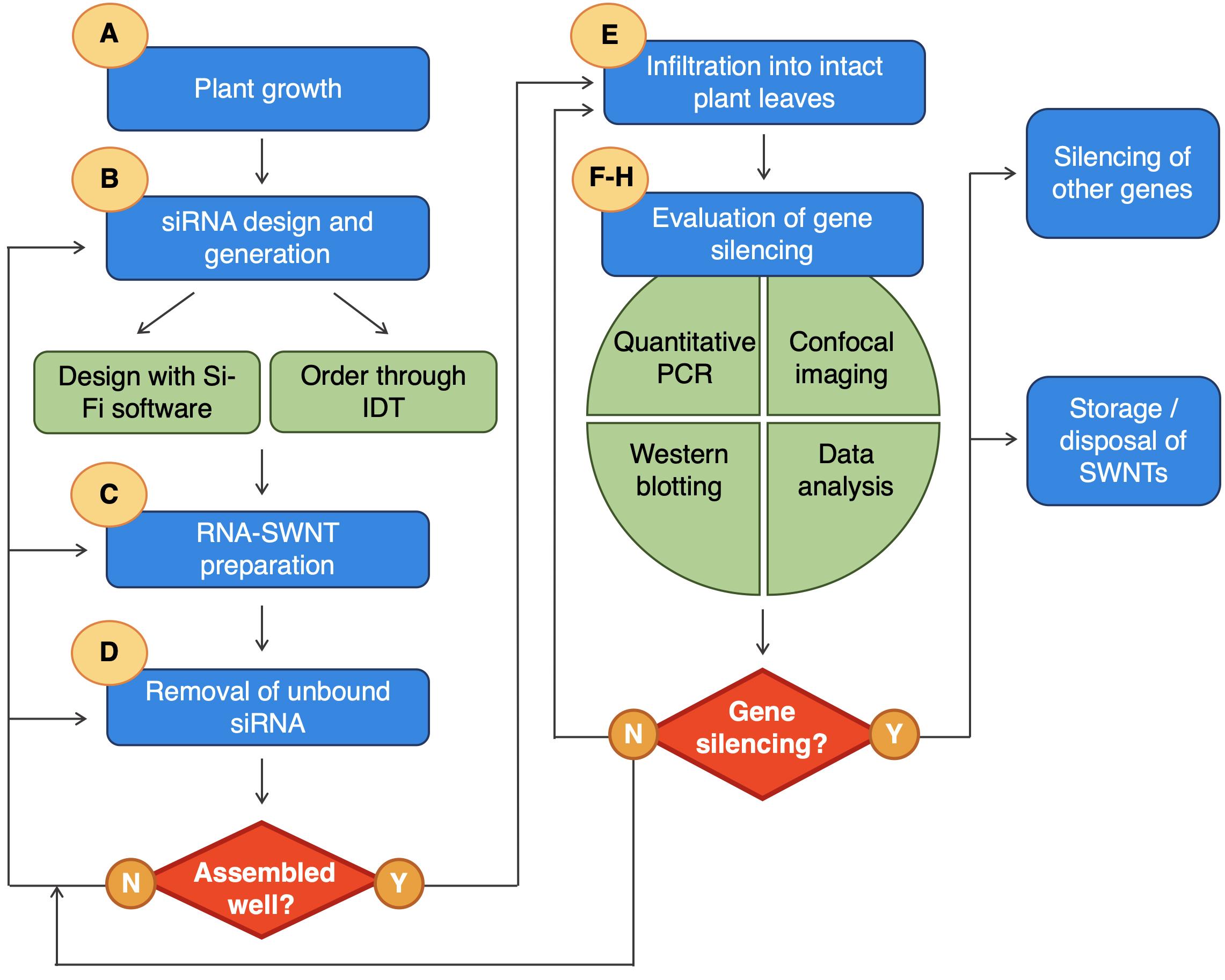
Figure 1. Overview of the siRNA-SWNT gene silencing procedure
Materials and Reagents
SunGro Sunshine LC1 Grower soil mix (SUN52128CFLP)
Delicate task wipes (Kimberly-Clark, catalog number: 06-666 )
100K MWCO Amicon spin filters (MilliporeSigma, catalog number: UFC510024 )
PVDF Membrane, Precut, 7 x 8.4 cm (Bio-Rad, catalog number: 1620174 )
Sterile syringe filter (0.45 μm; VWR, catalog number: 28145-481 )
Microcentrifuge tubes (1.5 ml; VWR, catalog number: 89000-028 )
Conical tubes (50 ml; Olympus, catalog number: 28-106 )
Pipette tips (Low retention 10 μl, 200 μl, 1,000 μl filter tips; USA Scientific, catalog numbers: 1181-3710 , 1180-8710 , 1182-1730 )
Extended-length pipette tips (1,000 μl; Eppendorf, catalog number: 00 30073614 )
#1 Microscopy cover glass (Fisher Scientific, catalog number: 12-542B )
Microscope slides (VWR, catalog number: 16004-422 )
Syringe (1 ml; BD, catalog number: 14-823-434 )
Mini Trans-Blot Filter paper (Bio-Rad, catalog number: 1703932 )
EasyStrip‚ Plus PCR Tube (Thermo Scientific, catalog number: AB2005 )
Plant seeds (mGFP5 Nicotiana benthamiana is obtained from the Staskawicz lab at UC Berkeley, mGFP5 plants constitutively express GFP targeted to the ER under the control of the Cauliflower mosaic virus 35S promoter)
Goat anti-rabbit horseradish peroxidase-conjugated antibody (Abcam, catalog number: ab205718 )
Anti-GFP antibody, ChIP Grade (Abcam, catalog number: ab290 )
HiPCO SWNTs (NanoIntegris, Super purified, catalog number: HS28-037 )
MilliQ water
Nuclease-free water (Qiagen, catalog number: 129114 )
Sodium chloride, NaCl (Sigma-Aldrich, catalog number: S9888-500G )
Hydrochloric acid, HCl (37% [vol/vol]; Sigma, catalog number: 320331 )
Single-stranded RNA oligonucleotides, including sense and antisense siRNA strands – 21 nucleotides (Integrated DNA Technologies, IDT)
Sodium dodecyl sulfate, molecular biology grade (Sigma-Aldrich, catalog number: 436143-100G )
Tris/HCl (Sigma-Aldrich, catalog number: 10812846001 )
EDTA (Sigma-Aldrich, catalog number: E9884-100G )
NP-40 (Sigma-Aldrich, catalog number: 492016-100ML )
Glycerol (Sigma-Aldrich, catalog number: G5516-500ML )
Pierce 660 nm Protein Assay (Thermo, catalog number: 22660 )
iScript cDNA synthesis kit (Bio-Rad, catalog number: 1708891 )
PowerUp SYBR green master mix (Applied Biosystems, catalog number: A25742 )
Qubit Protein Assay (ThermoFisher Scientific, catalog number: Q33211 )
RNeasy plant mini kit (QIAGEN, catalog number: 74904 )
BSA (Sigma-Aldrich, catalog number: A4737-25G )
TWEEN20 (Sigma-Aldrich, catalog number: P9416-100ML )
Ammonium persulphate, APS (Sigma, catalog number: 248614-100G )
Low range ultra agarose (Bio-Rad, catalog number: 1613107 )
ECL Prime Western Blotting System (MilliporeSigma, catalog number: GERPN2232 )
TEMED (N,N,N,N'-tetramethylethylenediamine; Sigma, catalog number: T9281 )
Glycine (Sigma, catalog number: G8898 )
Methanol (Sigma, catalog number: 179957 )
4x Laemmli sample Buffer (Bio-Rad, 10 ml, catalog number: 1610747 )
Liquid nitrogen
SYBR Gold Nucleic Acid Gel Stain (Invitrogen, catalog number: S11494 )
30% Acrylamide/Bis solution 19:1 (Bio-Rad, catalog number: 1610154 )
Protease inhibitor cocktail (Sigma, catalog number: P9599-1ML )
0.1 M NaCl (see Recipes)
10% (wt/vol) Ammonium persulphate solution (APS) (see Recipes)
10x Transfer buffer (see Recipes)
1x Transfer buffer (see Recipes)
10x Tris-Buffered Saline (TBS) buffer (1 M Tris, 1.5 M NaCl, pH 7.4) (see Recipes)
1x TBST buffer (see Recipes)
Lysis buffer (see Recipes)
Equipment
Analytical balance (Radwag, model: AS 60/220.R2 )
Ultrasonic bath (Branson, model: 15-336-100 )
Ultrasonic homogenizer with 6-mm tip (Cole-Parmer, models: UX-04711-70, UX-04712-14 )
Vortex mixer (Fisher Scientific, model: 02-215-365 )
pH meter (Spectrum, model: 242-97839 )
Orbital shaker (Waverly, model: S1CE )
NanoVue Plus spectrophotometer (GE Life Sciences, model: 28-9569-61 )
Visible spectrophotometer (Thermo Scientific, model: 14-385-445 )
Near-infrared spectrometer (Princeton Instruments IsoPlane 320 coupled to a liquid nitrogen-cooled Princeton Instruments PyLoN-IR 1D array of InGaAs pixels)
UV-Vis-NIR Spectrophotometer (Shimadzu, model: UV-3600 Plus )
Tabletop centrifuge (Eppendorf, catalog number: 5418000017 )
Centrifuge (Eppendorf, model: 5424R )
Tweezers (VWR, catalog number: 63042-518 )
Scissors (VWR, catalog number: 82027-582 )
Mortar and pestle (Cole-Parmer, catalog number: EW-63100-54 )
Pant growth chamber (HiPoint, model: 740 FHLED )
Gel image-analysis system ( Typhoon FLA 9500 , GE Healthcare Services)
Electrophoresis power supply (PowerPac basic power supply; Bio-Rad, catalog number: 1645050 )
Mini Trans-Blot Cell (Bio-Rad, catalog number: 1703811 )
Mini-Protein TGX gels (Bio-Rad, catalog number: 456-1094 )
ChemiDoc XRS+ System (Bio-Rad, catalog number: 1708265 )
Confocal Microscope (Zeiss, model: LSM 710 )
Thermal Cycler CFX96 Touch Real-Time PCR Detection System (Bio-Rad, catalog number: 1855195 )
Thermal Cycler PCR (Applied Biosystems Veriti 96-Well, catalog number: 4375786 )
Software
GraphPad Prism 7.0a (https://www.graphpad.com/scientific-software/prism/)
Fiji ImageJ 2.0.0 (https://imagej.net/Fiji/Downloads)
Zen Blue 2.6 (https://www.zeiss.com/microscopy/us/downloads.html)
Procedure
文章信息
版权信息
© 2021 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Demirer, G. S. and Landry, M. P. (2021). Efficient Transient Gene Knock-down in Tobacco Plants Using Carbon Nanocarriers. Bio-protocol 11(1): e3897. DOI: 10.21769/BioProtoc.3897.
分类
植物科学 > 植物分子生物学 > DNA > DNA 修饰
生物物理学 > 生物工程 > 纳米材料
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link











