- EN - English
- CN - 中文
Wounding Caenorhabditis elegans with Glass Wool
玻璃棉用于秀丽隐杆线虫的创伤研究
发布: 2021年01月20日第11卷第2期 DOI: 10.21769/BioProtoc.3885 浏览次数: 3249
评审: Ayush RanawadeMatthias RieckherAnonymous reviewer(s)
Abstract
Research on wound healing majorly relies on rat, mice and other animal models. However, an alternative animal model ought to be brought in the field, pertaining to the stringent ethical issues owing to the use of animals in research. In this regard, Caenorhabdits elegans, a miniature model nematode gains the great attention of the researchers in wound healing. Though, the model is being explored in wound research for more than a decade, the existing protocols lack the acquisition of large wound population that in turn could enable the utility of global genomics (G), proteomics (P) and metabolomics (M) based approaches. In order to overcome the inadequacy of the existing protocols, the protocol described here affords the acquisition of voluminous wound population in C. elegans using truncated glasswool pieces to enable the utility of high throughput analytical techniques.
Graphic abstract
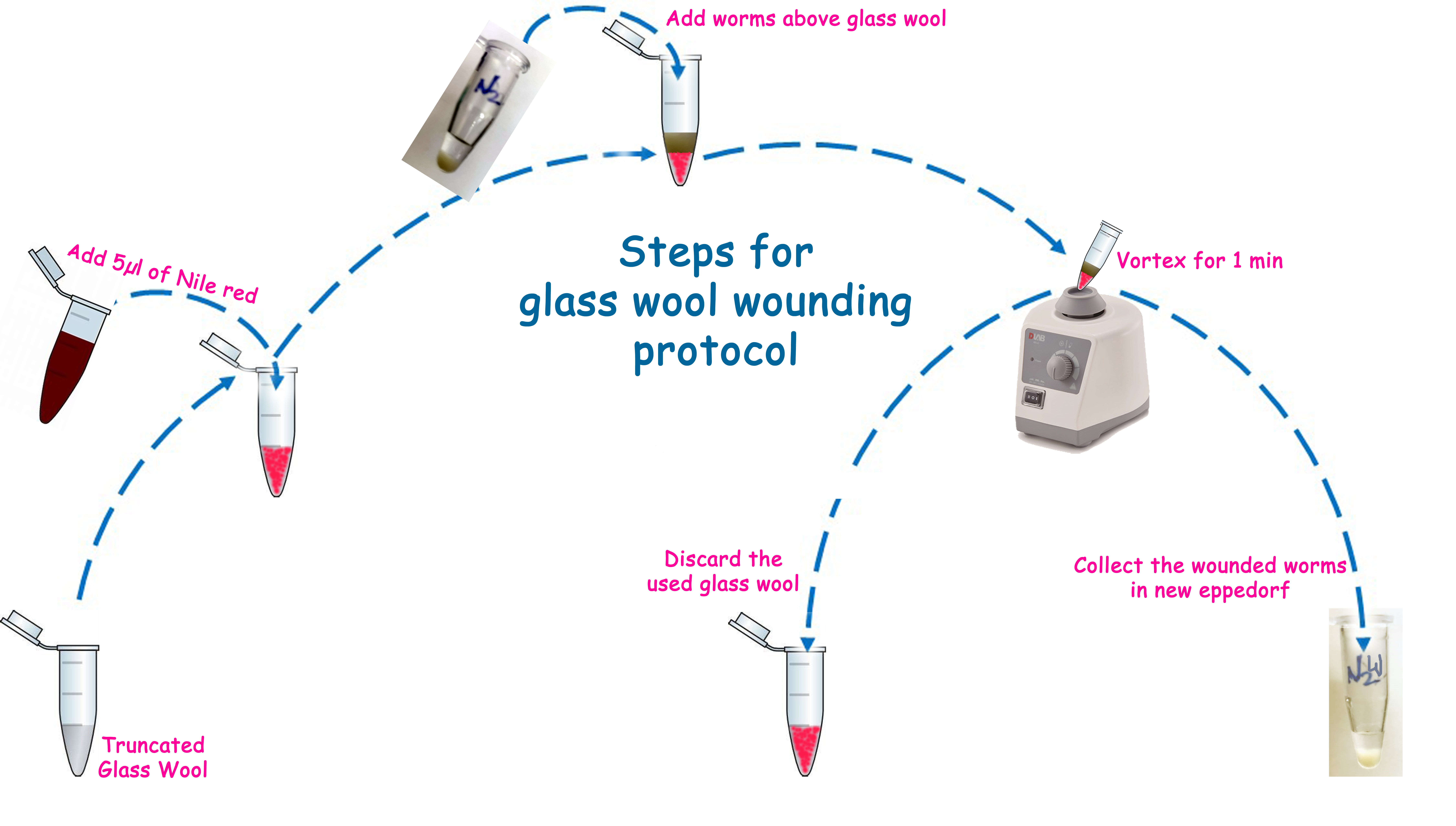
Steps involved in glass wool wounding protocol.
Background
Wounding of C. elegans is being done by fungal infection of Drechmeria coniospora (Pujol et al., 2008), micro-injection needle (Xu and Chisholm, 2014), femtosecond laser treatment (Chung et al., 2006) and micrometer-scale fine glass shards (Zhang et al., 2015). Most of these existing protocols involves individual wounding of worms in order to observe the roles of interested molecular players upon injury and during the process of healing. Existing methods have provided loads of new and useful insights from the mechanism of healing in C. elegans which was also proven to be extrapolatable to higher mammalian models. Nevertheless, the methods were inadequate to obtain large wound population in a shortframe of time and hence the further utility of high throughput alaytical techniques in G, P and M studies becomes tedious. In the era where high throughput analyses become the future of research, a new alternate protocol is required to obtain large wound population in single stretch at quick frame of time. Incidentally, glass wool wounding protocol described over here was established (Pooranachithra et al., 2019) inspired by the protocol described by Zhang et al., (2015). In addition to high throughput analytical techniques, the method was also found to be reliable for high throughput screening of active therapeutics for improved wound healing.
Materials and Reagent
- Glass wool (HIMEDIA, catalog number: RM1232)
- 50 ml Falcon tubes (TARSONS, catalog number: 546021 )
- 1.5 ml Eppendorf tubes (TARSONS, catalog number: 500010)
- 200 µl and 1 ml pipete tips (TARSONS, catalog numbers: 521010 and 521020)
- Microscope slides (LABTECH, catalog number: 217101) and Cover slips (BLUE STAR, 22 mm 10 g)
- 60 x 15 mm Petri plates
- C. elegans strain, N2 (Wild type) (CGC, Minnesota)
- E. coli OP50 (CGC, Minnesota)
- Nile red (Sigma, catalog number: 72485-100MG)
- Methanol (Fisher Scientific, catalog number: 43637G)
- KH2PO4
- K2HPO4
- MgSO4
- CaCl2
- Cholesterol
- Ethanol
- NaCl
- Peptone
- Agar
- Na2HPO4
- Tryptone
- Yeast extract
- House hold bleach solution
- KPO4 (1 M) (see Recipes)
- MgSO4 (1 M) (see Recipes)
- CaCl2 (1 M) (see Recipes)
- 5 mg/ml Cholesterol in 95% ethanol (see Recipes)
- NGM plates (see Recipes)
- M9 buffer (see Recipes)
- Nile red solution (see Recipes)
- LB broth (see Recipes)
Equipment
Scissors
T20, T200 and T1000 pipets (TARSONS, catalog numbers: 030020 ; 030040 ; 030050 )
Erlenmeyer flask (BOROSIL, catalog number: 4100029 )
Marter and Pestle (Thermo Scientific, catalog number: 533669 )
Dissecting stereo microscope (NIKON, model: SMZ 1000 )
20 °C C. elegans incubator (SANYO, Cooled incubator, model: MIR 554 )
37 °C bacterial incubator with shaker (SCIGENICS BIOTECH, ORBITEK, LETTD)
Vortex Mixer (DLAB, MX-S, 8031102000 )
Autoclave (EQUITRON, Vertical Autoclave)
Software
Microsoft Excel
Procedure
文章信息
版权信息
© 2021 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Pooranachithra, M., Bhaskar, J. P. and Balamurugan, K. (2021). Wounding Caenorhabditis elegans with Glass Wool. Bio-protocol 11(2): e3885. DOI: 10.21769/BioProtoc.3885.
分类
免疫学 > 动物模型 > 其它
细胞生物学 > 组织分析 > 损伤模型
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link

















