- EN - English
- CN - 中文
A Parkinson’s Disease-relevant Mitochondrial and Neuronal Morphology High-throughput Screening Assay in LUHMES Cells
LUHMES细胞中与帕金森氏病相关的线粒体和神经元形态学高通量筛选分析
发布: 2021年01月05日第11卷第1期 DOI: 10.21769/BioProtoc.3881 浏览次数: 5880
评审: Doyel SenOlga KopachAnonymous reviewer(s)
Abstract
Parkinson’s disease is a devastating neurodegenerative disorder affecting 2-3% of the population over 65 years of age. There is currently no disease-modifying treatment. One of the predominant pathological features of Parkinson’s disease is mitochondrial dysfunction, and much work has aimed to identify therapeutic compounds which can restore the disrupted mitochondrial physiology. However, modelling mitochondrial dysfunction in a disease-relevant model, suitable for screening large compound libraries for ameliorative effects, represents a considerable challenge. Primary patient derived cells, SHSY-5Y cells and in vivo models of Parkinson’s disease have been utilized extensively to study the contribution of mitochondrial dysfunction in Parkinson’s. Indeed many studies have utilized LUHMES cells to study Parkinson’s disease, however LUHMES cells have not been used as a compound screening model for PD-associated mitochondrial dysfunction previously, despite possessing several advantages compared to other frequently used models, such as rapid differentiation and high uniformity (e.g., in contrast to iPSC-derived neurons), and relevant physiology as human mesencephalic tissue capable of differentiating into dopaminergic-like neurons that highly express characteristic markers. After previously generating GFP+-LUHMES cells to model metabolic dysfunction, we report this protocol using GFP+-LUHMES cells for high-throughput compound screening in a restoration model of PD-associated mitochondrial dysfunction. This protocol describes the use of a robust and reproducible toxin-induced GFP+-LUHMES cell model for high throughput compound screening by assessing a range of mitochondrial and neuronal morphological parameters. We also provide detailed instructions for data and statistical analysis, including example calculations of Z’-score to assess statistical effect size across independent experiments.
Keywords: Parkinson’s disease (帕金森氏病)Background
Parkinson’s disease (PD) is a neurodegenerative disorder characterised primarily by loss of dopaminergic neurons in the substantia nigra of the midbrain and the accumulation of α-synuclein in intra-neuronal inclusions. It is the second most common neurodegenerative disorder affecting 2-3% of the population over 65 years of age (Poewe et al., 2017). Patients present with resting tremors, bradykinesia and muscle rigidity, along with non-motor symptoms which can include depression, anosmia and memory problems. There is currently no disease-modifying treatment that can prevent or slow the progression of PD, presenting an urgent need for an effective therapeutic (Armstrong and Okun, 2020).
Mitochondrial dysfunction is a key pathological hallmark of PD; many of the genetic loci associated with familial PD encode proteins that are involved in mitochondrial function or regulation (Hauser and Hastings, 2013). A variety of mitochondrial pathways are disturbed in PD pathology including ATP production, mitophagy, trafficking, biogenesis and calcium buffering (Park et al., 2018). It is therefore unsurprising that mitochondrial dysfunction is a popular target for therapeutic discovery in PD, which aims to identify compounds capable of enhancing those mitochondrial pathways disturbed in the disease state back towards normal physiological levels. This presents the challenge of finding an effective method to model mitochondrial dysfunction in vitro whereby large compound libraries can be screened efficiently within a disease-relevant model.
We have previously reported the use of primary patient fibroblasts to screen for compounds which rescue mitochondrial phenotypes seen in those fibroblasts (Mortiboys et al., 2013). Furthermore, recently a patient derived iPSC neuron model using a high-throughput, semi-automatic, imaging system was described to identify compounds which ameliorate mitochondrial clearance deficits (Yamaguchi et al., 2020). Both of these systems utilize patient derived cells, one potential issue when using patient derived models is the amount of material required and uniformity of sample. An alternative approach is the use of Lund human mesencephalic (LUHMES) cells. LUHMES cells are a subclone of the MESC2.10 cell line, derived from human embryonic ventral mesencephalic tissue and immortalised by integration of a v-myc retroviral factor which is tetracycline regulatable (Lotharius et al., 2002 and 2005). Briefly, LUHMES cells exist in a proliferative state until the addition of tetracycline, dibutyryl cyclic AMP (dCAMP) and glial cell derived neurotrophic factor (GDNF) which halts proliferation and induces uniform differentiation into a dopaminergic-like phenotype. As PD predominantly affects the dopaminergic neurons of the substantia nigra, the differentiated LUHMES cell phenotype is highly physiologically relevant as a model. Further characterisation has demonstrated that differentiated LUHMES cells resemble primary neuron cultures in many aspects: broad upregulation of neuronal markers, extensive neurite outgrowth and basic electrophysiological features (Scholz et al., 2011). Unlike many other transformed neuronal cell lines, LUHMES cells in the differentiated state have c-myc switched off and no dysregulation of the cell cycle is seen. This is particularly important when considering cell cycle mechanisms involved in neuronal DNA repair and neurodegeneration modelling. LUHMES cells are candidates for use in high-throughput screening due to their ease of handling, uniformity and high purity following differentiation induction (> 99%) compared to the often capricious and phenotypically variable culturing of primary neurons (Scholz et al., 2011). A comparative study also showed LUHMES cells express higher levels of neuronal markers (TUBB3, ENO2, MAP2) and have increased neurotoxicant sensitivity compared to two other commonly used neuronal models, SH-SY5Y neuroblastoma cells and human foetal neural stem cells (Tong et al., 2017).
LUHMES cells have been used extensively in high-throughput screening for the identification of neurotoxicants (Stiegler et al., 2011; Krug et al., 2013), including one specific to mitochondrial toxicity (Delp et al., 2019). However, we have only found one study to date that has exploited the high-throughput potential of LUHMES cells in PD therapeutic discovery. Höllerhage et al. used a previously developed alpha-synuclein LUHMES model to screen 1,600 FDA-approved drugs for protective effects by measuring cell viability after treatment (Höllerhage et al., 2017). Previously, we reported the generation of GFP+-LUHMES and the use of them to model metabolic dysfunction in co-culture with astrocytes (Ratcliffe et al., 2018).
Here we utilise the GFP+-LUHMES which we previously generated and validated as a useful model for metabolic dysfunction, to present a detailed and reproducible protocol for high-throughput screening of compounds in a toxin-induced differentiated LUHMES cell model relevant to PD. This protocol improves on previous studies by using high-content live cell imaging and analysis to assess mitochondrial parameters and neuronal morphology.
In the approach described here, differentiated LUHMES cells are treated with rotenone to model PD-associated mitochondrial dysfunction and neuronal loss in accordance with previous studies (Krug et al., 2013; Dolga et al., 2014). LUHMES cells are grown to full confluency before the addition of differentiation factors, and then replated into 384-well plates after two days. The cells remain in differentiation media for the full time course of the experiment. The plate is treated with rotenone on the fifth day, then treated with test compounds on the sixth day and live imaged on the seventh day (Figure 1). When designing a toxin induced model for screening; there are generally two paradigms. The first being a protection model, where the potential beneficial compound is added to the cells before the toxin. Secondly, and is the case in this protocol, is a restoration model; where the toxin is added first and then the potential beneficial compounds are added afterwards. This is testing the beneficial compounds to restore a defect already present as opposed to protecting from damage occurring. The advantage of this technique lies in the relative ease and speed in generating a uniform population of disease-relevant human dopaminergic neurons that can be used for large-scale compound screening. Furthermore, high-content live cell imaging and analysis allows the assessment of compound dose-response effects on a broad range of mitochondrial and neuronal morphological parameters.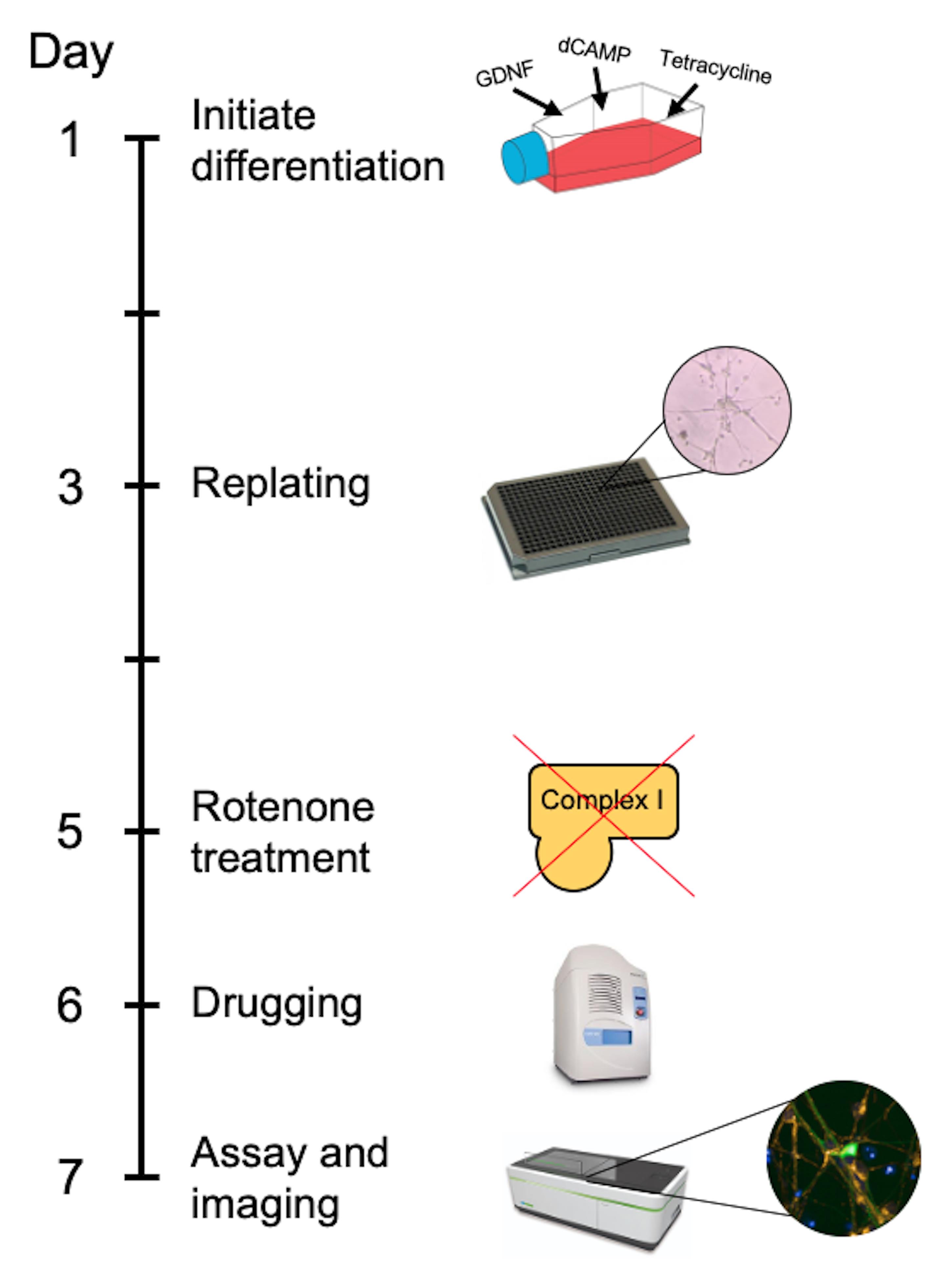
Figure 1. Timeline of the GFP-LUHMES drug screening protocol
Materials and Reagents
Materials
384-well black plates (Greiner-Bio, catalog number: 781091 )
384-well LDV source plates (Labcyte, catalog number: LP-0200 )
384-well source plate seals (Fluidx, catalog number: 41-1011 )
5 ml serological pipettes (Fisher Scientific, catalog number: 13-676-10H )
10 ml serological pipettes (Fisher Scientific, catalog number: 13-676-10J )
25 ml serological pipettes (Fisher Scientific, catalog number: 13-678-11 )
10 μl pipette tips (Fisher Scientific, catalog number: 02-707-441 )
200 μl pipette tips (Fisher Scientific, catalog number: 02-707-422 )
1,000 μl pipette tips (Fisher Scientific, catalog number: 02-707-402 )
15 ml Falcon tubes (Greiner-Bio, catalog number: 188271 )
50 ml Falcon tubes (Greiner-Bio, catalog number: 227261 )
T75 flasks (Greiner-Bio, catalog number: 658175 )
Cryovials (Greiner-Bio, catalog number: 122261 )
Reagents
LUHMES cells (ATCC® CRL-2927TM)
GFP-LUHMES cells (RRID: CVCL_B056, Ratcliffe et al., 2018)
GFP-expressing lentiviral particles
Advanced DMEM/F-12 (Thermo Scientific, catalog number: 12634010 )
Fibronectin (Sigma-Aldrich, catalog number: FC010 )
Poly-L-Ornithine (Sigma-Aldrich, catalog number: P4957 )
PBS Tablets (Thermo Scientific, catalog number: BR0014G )
Trypsin 10x (Lonza, catalog number: BE02-007E )
L-Glutamine (Lonza, catalog number: BE-17-605E )
N-2 Supplement (Gibco, catalog number: 11520536 )
Pen-Strep (Lonza, catalog number: DE17-603E )
FGF-basic (Peprotec, catalog number: 100-18B )
dCAMP (Sigma-Aldrich, catalog number: D0627 )
GDNF (Peprotech, catalog number: 450-10 )
Tetracycline (Sigma-Aldrich, catalog number: T7660 )
Hoechst (Sigma, catalog number: 94403 )
TMRM (Invitrogen, catalog number: T668 )
Sterile 1x PBS (see Recipes)
Sterile 1x Trypsin (see Recipes)
LUHMES Base Media (see Recipes)
Advanced DMEM/F-12
N-2 Supplement
Pen-Strep
L-Glutamine
LUHMES Proliferation Media (see Recipes)
LUHMES Base Media
FGF-basic
LUHMES Differentiation Media
Tetracycline
dCAMP
GDNF
Equipment
Mechanical pipette gun for serological pipettes
Mechanical pipettes (P20, P200, P1000)
Multichannel mechanical pipettes (P10, P200)
4 °C fridge
-20 °C freezer
-80 °C freezer
Sterile tissue culture hood
Autoclave
Centrifuge for tissue culture (MSE Harrier 15/80, catalog number: MSB080.CX1.5 )
Centrifuge for source plates
CO2 Incubator (Sanyo, model: MCO-19AIC )
Echo 550 Liquid Handler (Labcyte, catalog number: Echo 550 )
MultiPod Controller (Roylan Developments, catalog number: SPOD0012 )
StoragePod Enclosure (Roylan Developments, catalog number: SPOD0010 )
Opera Phenix High-Content Screening System (PerkinElmer)
Software
Echo Liquid Handler Software (Labcyte)
Echo Plate Reformat (Labcyte)
Harmony High-Content Imaging and Analysis Software (PerkinElmer)
Columbus Image Data Storage and Analysis System (PerkinElmer)
Excel 2016 (Microsoft)
GraphPad Prism 8.2 (GraphPad)
Procedure
文章信息
版权信息
© 2021 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Leah, T., Vazquez-Villaseñor, I., Ferraiuolo, L., Wharton, S. B. and Mortiboys, H. J. (2021). A Parkinson’s Disease-relevant Mitochondrial and Neuronal Morphology High-throughput Screening Assay in LUHMES Cells. Bio-protocol 11(1): e3881. DOI: 10.21769/BioProtoc.3881.
分类
细胞生物学 > 细胞成像 > 活细胞成像
神经科学 > 发育 > 神经元
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link