- EN - English
- CN - 中文
Lipid Droplet Isolation from Arabidopsis thaliana Leaves
拟南芥叶脂滴的分离
(*contributed equally to this work) 发布: 2020年12月20日第10卷第24期 DOI: 10.21769/BioProtoc.3867 浏览次数: 4570
评审: Agnieszka ZienkiewiczMagdalena Miklaszewska Amelie Kelly
Abstract
Lipid droplets (LDs) are neutral lipid aggregates surrounded by a phospholipid monolayer and specific proteins. In plants, they play a key role as energy source after seed germination, but are also formed in vegetative tissues in response to developmental or environmental conditions, where their functions are poorly understood. To elucidate these, it is essential to isolate LDs with good yields, while retaining their protein components. LD isolation protocols are based on their capacity to float after centrifugation in sucrose gradients. Early strategies using stringent conditions and LD-abundant plant tissues produced pure LDs where core proteins were identified. To identify more weakly bound LD proteins, recent protocols have used low stringency buffers, but carryover contaminants and low yields were often a problem. We have developed a sucrose gradient-based protocol to isolate LDs from Arabidopsis leaves, using Tween-20 and fresh tissue to increase yield. In both healthy and bacterially-infected Arabidopsis leaves, this protocol allowed to identify LD proteins that were later confirmed by microscopy analysis.
Keywords: Lipid droplet isolation (脂滴分离)Background
Lipid droplets (LDs) are organelles composed by a neutral lipid core surrounded by a phospholipid monolayer where LD proteins are inserted (Tzen et al., 1993). LDs were first described as stable compartments for storage of high-energy lipids in specialized tissues. However, recent reviews describe LDs as highly dynamic organelles present in most cell types, with functions that transcend mere energy storage (Shimada et al., 2018; Huang, 2018; Shao et al., 2019).
In plants, early studies on LDs were conducted mostly in oilseeds, where they are very abundant and act as energy source in the post-germination stage, before photosynthetic capacity is acquired (Yatsu and Jacks, 1972; Huang, 1996; Tzen et al., 1997). Subsequent works revealed that these organelles are also formed in pollen grains (Ischebeck, 2016), senescent leaves (Brocard et al., 2017) and pathogen-infected tissues (Shimada et al., 2014; Fernández-Santos et al., 2020), although their functions are poorly understood. It is therefore imperative to have efficient protocols for LD isolation from vegetative tissues, where their abundance is comparatively low.
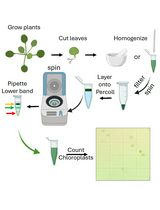
LDs are the least dense cellular organelles, hence, most isolation protocols are based on their capacity to float after centrifugation. Tissue is homogenized in a suitable buffer, and then the extract is clarified and centrifuged with an overlay of a less dense buffer (floating buffer). Upon centrifugation, LDs float from the extraction buffer and through the floating buffer, to accumulate in a fat pad visible on top of the tube.
The choice of floating and extraction buffers poses a dilemma to the experimenter. Their stringency and the number of centrifugations will establish the balance between obtaining cleaner LDs, but devoid of some loosely bound, bona fide LD proteins, or more “complete” LDs with carryover contaminants attached during preparation (Huang, 2018). Early protocols from oilseeds often included harsh buffers, containing detergents, organic solvents, chaotropic agents or high salt concentrations (Tzen et al., 1997; Jolivet et al., 2004; Buchanan-Wollaston et al., 2005; Katavic et al., 2006). As LDs are very abundant in this tissue, such conditions guaranteed high yields of pure LDs where core proteins were identified (Tzen et al., 1990; Chen et al., 1999; Lin et al., 2002).
In order to identify less tightly bound LD proteins, later protocols designed for proteomics applications have tended to less stringent conditions, as in Horn et al. (2013) and Brocard et al. (2017). In these cases, an enrichment factor was calculated for each protein between LDs and other fractions to rule out contaminants. “True” LD proteins were thus defined as those above an arbitrary threshold. However, proteins with dual localization can be missed with this strategy, especially if the studied condition is dynamic and LD formation is transient. Unfortunately, there is no perfect negative control, and “true” LD components will always need to be confirmed by complementary methods such as microscopy. Another problem in previous protocols from leaves has been the large amount of sample needed for extraction, due to lower LD abundance in this tissue compared with seeds or fruit mesocarp (Brocard et al., 2017).
Based on this background, we developed an LD isolation protocol from Arabidopsis leaves, with the following aims: i) simplicity ii) maximization of LD yield and iii) low to medium stringency in order to identify loosely bound LD proteins while minimizing contaminants. Briefly, we extract LDs in a 0.6M sucrose buffer with 0.1% Tween-20 followed by two successive flotations in the same buffer with less sucrose (0.4 M and 0.2 M). The amount of Tween-20 can be variable and is critical in two aspects: increasing stringency and LD yield. The other critical point in our hands was to use fresh tissue, as yield and integrity of LDs is affected when extracted from frozen samples. We obtained approx. 1,700 LDs/mg fresh tissue using 6-week-old wild type plants and were able to identify and confirm known LD proteins such as CLO3 and α-DOX1, as well as proteins newly associated to LDs in plants such as GPAT4, GPAT8 and PAD3 (Fernández-Santos et al., 2020). LD quality can be checked by microscopy or flow cytometry. By using transgenic CLO3:GFP Arabidopsis plants, we were also able to follow the isolation process by western blot using an anti-GFP antibody.
Materials and Reagents
Glass Pasteur pipettes (Deltalab, catalog number: 702 )
5 ml Open-top polypropylene tubes (Beckman Coulter, catalog number: 326819 )
(Optional) 13 ml Open-top polypropylene tubes (Beckman Coulter, catalog number: 331372 )
(Optional) Liquid nitrogen
Miracloth (Merck Milipore, catalog number: 475855-1R )
Sea sand (Merck, catalog number: 1.07712.1000 )
Sucrose (Carlo Erba Reagents, catalog number: 365157 )
NaH2PO4.H2O (Merck, catalog number: 1.06346.0500 )
NaCl (VWR chemicals, catalog number: 27810.364 )
NaOH (AppliChem, catalog number: 131687.1211 )
Tween-20 (Sigma Aldrich, catalog number: P1379-500ml )
PMSF (Sigma Aldrich, catalog number: 93482-50ml-F )
CompleteTM Protease Inhibitor Cocktail (Roche, catalog number: 11697498001 )
BODIPY493/503 (Thermo Fisher, catalog number: D3922 )
Nile Red (Thermo Fisher, catalog number: N1142 )
DMSO (Merck, catalog number: 1.02952.1000 )
Methanol (Sigma Aldrich, catalog number: 32213-2.5L-M )
(Optional) 35S::CLO3:GFP transgenic Arabidopsis plants
Base buffer (600 ml) (see Recipes)
Sucrose buffers (100 ml each) (see Recipes)
1.2 M Sucrose buffer
0.8 M Sucrose buffer
0.4 M Sucrose buffer
Supplemented base buffer (300 ml) (see Recipes)
Phosphate buffer (100 ml) (see Recipes)
Stocks (see Recipes)
1 mg/ml BODIPY495/503
1 mg/ml Nile Red
50 mM PMSF
Equipment
P1000 pipette
Funnel
Mortar and pestle
Scissors
Ultracentrifuge (Beckman Coulter, model Optima L-100 XP )
Rotor SW55 Ti (Beckman Coulter, catalog number: 342194 )
(Optional) Rotor SW41 Ti (Beckman Coulter, catalog number: 331362 )
Fluorescence microscope (Leica, model TCS-SP8 )
pH Meter (Hach, model SensIONTM pH 3, catalog number: LPV2000.98.0002 )
(Optional) Flow cytometer (Beckman Coulter, model GaliosTM)
(Optional) Standard SDS-PAGE and Western blot equipment
Procedure
文章信息
版权信息
© 2020 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Izquierdo, Y., Fernández-Santos, R., Cascón, T. and Castresana, C. (2020). Lipid Droplet Isolation from Arabidopsis thaliana Leaves. Bio-protocol 10(24): e3867. DOI: 10.21769/BioProtoc.3867.
分类
植物科学 > 植物细胞生物学 > 细胞器分离
细胞生物学 > 细胞器分离 > 脂肪体
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link