- EN - English
- CN - 中文
Magnet-assisted Flow Cytometry of in vivo Tumors to Quantitate Cell-specific Responses to Magnetic Iron Oxide Nanoparticles
磁辅助流式细胞术检测体内肿瘤细胞以量化对磁性氧化铁纳米粒子的特异性反应
发布: 2020年11月20日第10卷第22期 DOI: 10.21769/BioProtoc.3822 浏览次数: 3908
评审: Anonymous reviewer(s)
Abstract
A clear understanding of nanoparticle interactions with living systems at the cellular level is necessary for developing nanoparticle-based therapeutics. Magnetic iron oxide nanoparticles provide unique opportunities to study these interactions because of their responsiveness to magnetic fields. This enables sorting of cells containing nanoparticles from in vivo models. Once sorted, flow cytometry can identify individual cell types, which can be further analyzed for iron content, gene or protein expression changes associated with nanoparticle uptake, and for other biological responses at a molecular level. Here we provide a detailed protocol to sort and identify cells in the tumor microenvironment that have internalized magnetic iron oxide nanoparticles following intravenous administration.
Keywords: Flow cytometry (流式细胞术)Background
Several nanoparticle-based cancer medicines are in clinical use because they have demonstrated safety and efficacy for diagnosis, cancer drug delivery, and hyperthermia (Marchal et al., 2015; Wu et al., 2015). Yet, there remain critical gaps in our understanding of cancer nanomedicine, largely because details of interactions between nanoparticles and immune cells remain unknown. Others and we have identified nanoparticle-immune cell interactions as critical variables affecting in vivo nanoparticle fate and retention in tumors (Sheen et al., 2014; Zanganeh et al., 2016; Korangath et al., 2020). Flow cytometry enables quantitative analytical sorting of mixed cell populations by combining light scattering with detection of a fluorescent signal from appropriately labeled cells. To track nanoparticle uptake in cells, the nanoparticles are often labeled with a fluorescent dye to enable detection. The presence of dyes or other ‘excipient’ molecules on nanoparticles can change nanoparticle pharmacokinetics and biodistribution in unintended and unrealized ways, and do not provide sufficient analyte for quantitative validation of nanoparticle concentration. Here we demonstrate the use of the magnetic property of unlabeled iron oxide nanoparticles (no fluorescence labeling) taken up by the cells, when administered intravenously. Our developed assay provides quantitative and cell-specific data of unaltered nanoparticle uptake following systemic (i.e., intravenous) delivery. Our assay exploits the directional response of magnetic iron oxide nanoparticles (MIONs) to high-gradient magnetic fields, which causes cells containing the MIONs to sediment near the magnet, whereas cells devoid of MIONs remain in supernatant. In this manner, cells are ‘pre-sorted’ by magnetic separation to differentiate between the two populations before flow cytometry analysis. Following sorting, cells can be analyzed further by inductively coupled plasma mass spectrometry (ICP MS) or ferene-s assay (Hedayati et al., 2018) to validate concentration of the analyte (Fe) which is then related to intracellular nanoparticle content; and, by other gene- or protein-based array technologies to assess biological responses at the molecular level within cells (Figure 1).
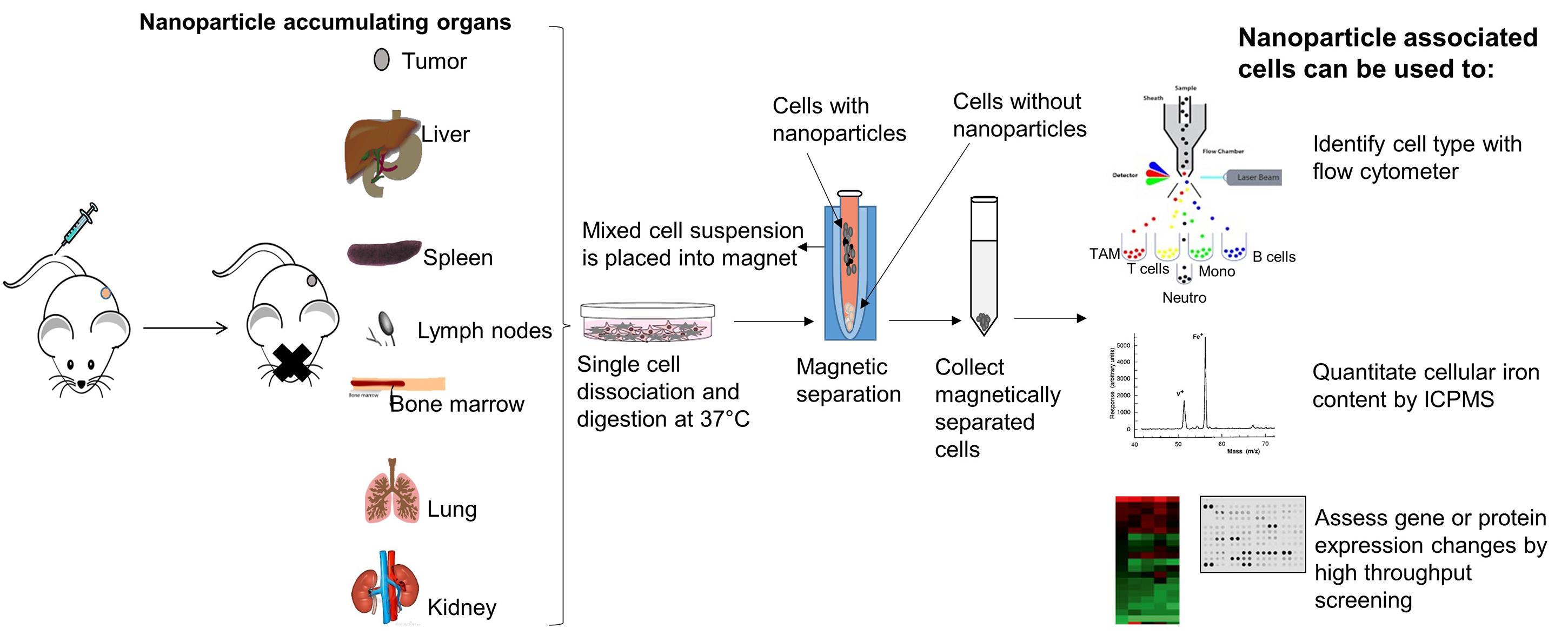
Figure 1. Concept of magnet-assisted cell isolation for detailed downstream analysis. When injected into the tail vein, iron oxide nanoparticles accumulate in organs and tumor. After harvest, tissues can be dissociated into single cells and nanoparticle-associated cells can then be isolated by placing the entire mixture on a permanent rare earth magnet. Cells associated with the iron oxide nanoparticles (internal or membrane bound) will separate from the suspension and attach to the tube wall proximal to the magnet. Cells not associated with a significant concentration of nanoparticles will remain in suspension (supernatant). Magnetically sorted cells can then be further analyzed with a flow cytometer to distinguish among cell types or lineages. Absolute amount of iron in cells can be determined by inductively coupled plasma mass spectrometry (ICPMS), or by other quantitative iron assays. Nanoparticle-induced changes in cells at the molecular level can be identified by further analysis using gene- or protein-based array technologies. Comparisons with cells not associated with the nanoparticles is also possible by collecting and analyzing the supernatant.
Materials and Reagents
Micropipette tips (Rainin, catalog numbers: 17005860 [20 µl]; 30389240 [200 µl]; 30389213 [1,000 µl])
Corning® non-treated culture dishes (D × H 100 mm × 20 mm) (Corning, catalog number: 430591 )
Syringe (3 ml) (Fisher Scientific, catalog number: 14-823-436 )
Conical tube (50 ml) (Thermo Scientific, catalog number: 339653 )
Cell strainer Nylon (100 µm) (BD Falcon, catalog number: 352360 )
Aluminum foil
IntegraTM MiltexTM Sterile Surgical Blades (Fisher Scientific, catalog number: 12-460-444 )
Microcentrifuge tubes (Fisher Scientific, catalog number: 05-408-137 )
FalconTM Round-Bottom Polystyrene Tubes (Flow tubes) (Corning, catalog number: 352054 )
ACK lysing buffer (Quality Biologicals, catalog number: 118-156-101 )
Dulbecco’s Modified Essential Medium (DMEM) high glucose (MilliporeSigma, catalog number: D5796 )
Heat Inactivated FBS (HI-FBS) (Fisher Scientific, catalog number: SH30109.03HI )
Collagenase Type 4 (Worthington Biochemical Corporation, catalog number: CLS4 )
Hyaluronidase (MP Biomedicals, catalog number: 0 2151272 )
Phosphate buffered saline (PBS) (Corning Cellgro, catalog number: 21-040-CV )
OneComp eBeads (eBioscience, catalog number: 01-1111-42 )
eBioscienceTM Fixation/Permeabilization Concentrate (Thermo Scientific, catalog number: 00-5123-43 )
eBioscienceTM Fixation/Permeabilization Diluent (Thermo Scientific, catalog number: 00-5223-56 )
Anti CD16/32 antibody (Fc blocker) (BioLegend, catalog number: 101302 ), dilution 1:100
Zombie Aqua (Live/dead stain) (BioLegend, catalog number: 423102 ), dilution 1:100
Anti CD45- APC-Cy7 antibody (BioLegend, catalog number: 103116 ), dilution 1:500
Anti MHCII- AF700 antibody (BioLegend, catalog number: 107622 ), dilution 1:200
Anti CD3- BV711 antibody (BioLegend, catalog number: 100241 ), dilution 1:500
Anti TCRγδ- PE antibody (BioLegend, catalog number: 118108 ), dilution 1:1,000
Anti CD19- BV650 antibody (BioLegend, catalog number: 115541 ), dilution 1:500
Anti CD49b- PERCP Cy5.5 (BioLegend, catalog number: 108916 ), dilution 1:200
Anti CD11b- BV421 antibody (BioLegend, catalog number: 101235 ), dilution 1:200
Anti Ly6C- APC antibody (BioLegend, catalog number: 128016 ), dilution 1:200
Anti Ly6G- FITC antibody (BioLegend, catalog number: 127606 ), dilution 1:500
Anti F4/80- BV605 antibody (BioLegend, catalog number: 123133 ), dilution 1:200
Anti CD11c- PECy7 antibody (BD Pharmingen, catalog number: 561022 ), dilution 1:500
Anti Cytokeratin-PE antibody (Abcam, catalog number: Ab52460 ), dilution 1:500
Ice
Dimethyl sulfoxide (DMSO)
Equipment
Micropipettes
Tube holders and racks
Ice holder
Biosafety cabinet (BSL2)
Rotator (Fisher Scientific, UVP HB-1000 Hybrodizer Hybridization Oven, catalog number: UVP 95003001 )
Centrifuge (Eppendorf, model: 5810 R )
Rare earth permanent magnet (Promega, Magnesphere® Technology magnetic separation stand, catalog number: Z5343 )
Incubator 37 °C with 5% CO2 (Thermo Scientific, Heracell 160I, model: 41774412 )
Nexcelom cell counter (Nexcelom Bioscience, Cellometer auto T4 automated cell counter, model: Auto T4-003-0489 )
Flow cytometer (BD Biosciences, model: LSR-II )
Software
BD FACSDiva software (BD Biosciences, CA, USA)
Flowjo software version 10 (BD Biosciences, CA, USA)
Procedure
文章信息
版权信息
© 2020 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Korangath, P. and Ivkov, R. (2020). Magnet-assisted Flow Cytometry of in vivo Tumors to Quantitate Cell-specific Responses to Magnetic Iron Oxide Nanoparticles. Bio-protocol 10(22): e3822. DOI: 10.21769/BioProtoc.3822.
- Korangath, P., Barnett, J. D., Sharma, A., Henderson, E. T., Stewart, J., Yu, S. H., Kandala, S. K., Yang, C. T., Caserto, J. S., Hedayati, M., Armstrong, T. D., Jaffee, E., Gruettner, C., Zhou, X. C., Fu, W., Hu, C., Sukumar, S., Simons, B. W. and Ivkov, R. (2020). Nanoparticle interactions with immune cells dominate tumor retention and induce T cell-mediated tumor suppression in models of breast cancer. Sci Adv 6(13): eaay1601.
分类
癌症生物学 > 微环境
免疫学 > 免疫细胞功能
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link













