- EN - English
- CN - 中文
Long-distance Transport in Bacterial Swarms Revealed by Single Nanoparticle Tracking
通过单纳米粒子追踪揭示细菌集群的长距离运输
发布: 2020年11月05日第10卷第21期 DOI: 10.21769/BioProtoc.3812 浏览次数: 3212
评审: David PaulArvind Kumar Singh ChandelAnonymous reviewer(s)
Abstract
During swarming, high density flagella-driven bacteria migrate collectively in a swirling pattern on wet agar surfaces, immersed in a thin viscous fluid layer called “swarm fluid”. Though the fluid environment has essential role in the emergence of swarming behavior, the microscopic mechanisms of it in mediating the cooperation of bacteria populations are not fully understood. Here, instead of micro-sized tracers used in previous research, we use gold nanorods as single particle tracers to probe the dynamics of the swarm fluid. This protocol includes five major parts: (1) the culture of swarming bacterial colony; (2) the preparations of gold nanorod tracers and the micro-spraying technique which are used to put the nanotracers into the upper fluid of bacterial swarms; (3) imaging and tracking; (4) other necessary control experiments; (5) data analysis and fitting of physical models. With this method, the nano-sized tracers could move long distances above motile cells without direct collisions with the bacteria bodies. In this way, the microscopic dynamics of the swarm fluid could be tracked with high spatiotemporal resolution. Moreover, the comprehensive analysis of multi-particle trajectories provides systematic visualization of the fluid dynamics. The method is promising to probe the fluid dynamics of other natural or artificial active matter systems.
Keywords: Collective motion (集体运动)Background
Recently, collective motions of bacteria have attracted a lot of attention in the field of active matter/fluid (Rabani et al., 2013 ; Zhang, H. et al., 2010; Marchetti et al., 2013). Due to the self-driven motion of individual bacterium propelled by flagella, high density bacteria suspensions would fall into a non-equilibrium state and emerge collective structures such as vortices, jets ( Mendelson et al., 1999 ) or turbulences (Wensink et al., 2012 ) in a scale far larger than the size of an individual bacterium. As one of the most widely used model system for the study of bacteria collection motions (Copeland and Weibel, 2009), bacteria swarming refers to coordinated migrations of cells across wet solid agar surfaces with swirling patterns (Kearns, 2010). During swarming, motile cells are immersed in a thin layer of highly viscous fluid called “swarm fluid” (Wu and Berg, 2012). Physically, the interplay between the fluid medium and the stirring flagella provides the dynamic origins of the collective motion though far-field hydrodynamic interactions (Koch and Subramanian, 2011). Biologically, the fluid environment enhances the mixing and diffusion of oxygen, nutrition as well as the signaling molecules involved in Quorum sensing (Hardman et al., 1998 ) and chemotaxis (Taktikos et al., 2012 ). Although the swarm fluid is important for the generation of large-scale synergetic patterns, how it works and its relationships with fast moving cells remain unclear especially at the microscopic scale.
Single particle tracking has been regarded as a powerful technique to investigate the complex fluid (Burov et al., 2011 ). Previous studies have used micro-sized particles as single particle tracers to characterize the properties of swarm fluid. Wu et al. used 1~2 µm micro-bubbles produced by explosive transformation of the water insoluble surfactant Span-83 droplets as tracers to reveal the intensive matter transfer flow in the leading edge of the swarming colony (Wu et al., 2011). Zhang et al. fabricated MgO particles as tracer particles through burning magnesium ribbons. They found that 0.2 µm MgO particles only diffused normally within a small region (~4 µm2) as the swarm front approaches (Zhang, R. et al., 2010). However, these methods of producing tracer particles are complicated and difficult to control. In addition, the prepared gold nanorods are often uneven in size. On the other hand, micro-sized tracers with various sizes and materials would either inevitably collide with bacteria bodies or be trapped at the liquid-air surface, making the tracers’ motions incapable of revealing real dynamics of the fluid motions. Plasmonic gold nanorods (AuNRs) have long been used as nanotracers for long-duration observations in biological studies with high spatio-temporal resolution. The AuNRs have good photostability and low cytotoxicity. Moreover, the methods of preparing stable, uniform, and monodispersed AuNRs are very mature. Here, with Bacillus subtilis as a model strain, we introduced 40 x 84 nm AuNRs as tracers into the bacterial swarm fluid. The AuNRs tracers could move freely in the upper layer without obvious physical contacts with the cell bodies. Combined with high spatiotemporal resolution of imaging and tracking technique, the trajectories of multi-nanoparticles in the large field of view could be obtained. By detailed diffusion analysis of tracers’ trajectories, we found that the swarm fluid could transport the AuNRs to long distances in a super diffusive, Lévy mode (Figure 1). This result is consistent with previous studies that individual bacterium in swarming would exhibit characteristic motions of Lévy walk ( Ariel et al., 2015 ).
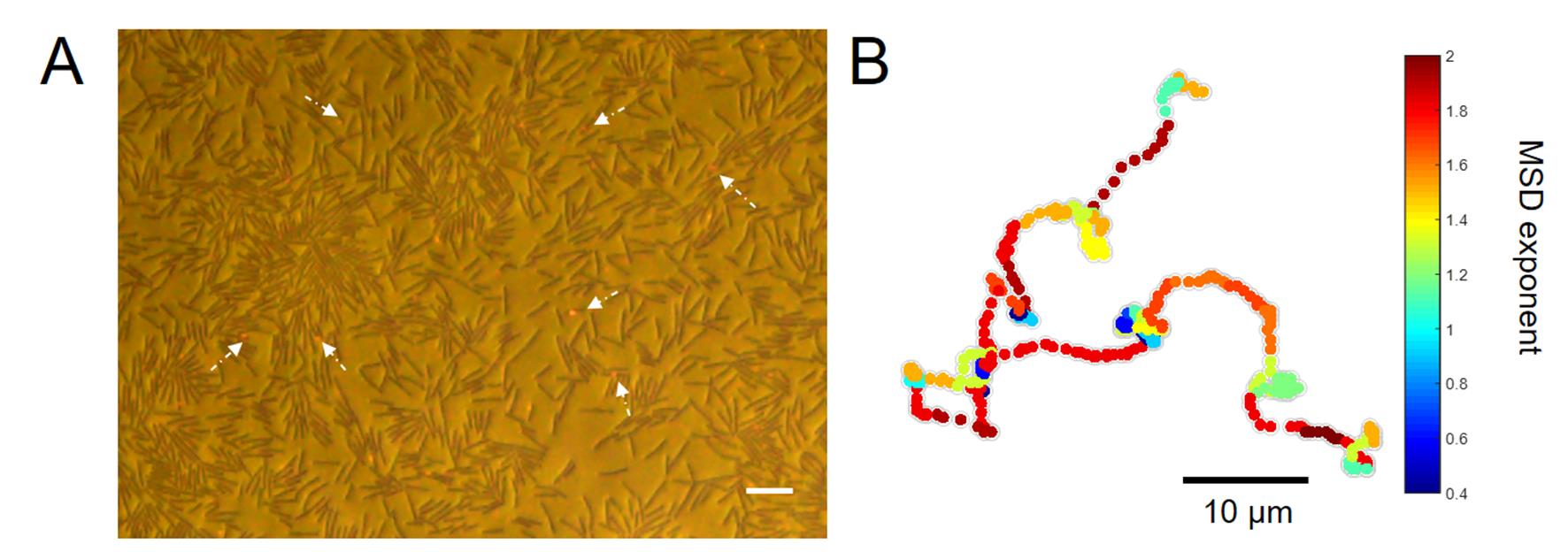
Figure 1. The gold nanorods move in high-efficiency on the upper fluid layer of the swarming bacteria. A. A captured image of the swarming bacteria and AuNRs. The brown rods are bacteria. The red spots which are indicated by white arrows are AuNRs. The scale bar is 10 μm. B. A typical trajectory illustrates that AuNRs move in a super-diffusive, Lévy mode with heterogeneous MSD local exponents.
Materials and Reagents
Plastic Petri dishes, 60 mm and 90 mm. Brand is not critical
Serum bottles, 250 and 500 ml (brand is not critical)
Note: Ensure that the bottles could withstand temperatures of at least 121 °C.
Square cover glass slides, 22 x 22 mm (Corning, catalog number: 2870-25 )
Single concave microscope glass slide, 25.4 x 76.2 mm (Sail Brand, catalog number: CAT.NO. 7101 )
Disposable sterile syringe filters, 0.22 µm diameter (Bioland, catalog number: PT13-022 )
230 mesh carbon film, copper grids (Zhongjing Keyi, catalog number: BZ110223b )
Bacillus subtilis NCIB 3610 (purchased from China General Microbiological Culture Collection center, catalog number: 1.3358 )
Tryptone, Bacto (BD Difco, catalog number: 211705 )
Yeast extract, LD, Bacto (BD Difco, catalog number: 210933 )
Bacto Agar (BD Difco, catalog number: 214010 )
NaCl (Beijing Chemical Works, catalog number: A1060005 )
LB agar, powder (Solarbio, catalog number: L1015 , storage: 2-8 °C)
SH-PEG modified gold nanorods 40 x 84 nm (NanoSeedz, catalog number: PEG-40-650-50 )
COOH-modified polystyrene microspheres, 0.5 µm (Dae technique, catalog number: PSC-00500 )
Gold nanospheres 90 nm and 120 nm
Note: We synthesized the gold nanospheres according to the methods in the reference paper (Zhou et al., 2011).
Milli-Q H2O (from Milli-Q Water Purification System, Millipore Sigma)
Soft LB agar for swarming (see Recipes)
LB broth, solution (see Recipes)
Equipment
Micro pipettes, 5, 10, 100, 200 and 1,000 μl (Eppendorf)
Autoclave (purchased from Alibaba) (brand is not critical)
Microwave oven (Meidi) (brand is not critical)
4 °C and -80 °C refrigerator
Shaker incubator (Jiecheng Experimental Apparatus, model: TS-100B )
Constant temperature and humidity incubator (Yiheng Instrument, model: LHS-80HC- )
UV-visible spectrophotometer (Shimadzu Corporation, model: UV1800 )
Hand ultraviolet ray examining lamp, 365 nm (Yuhua Instrument, model: ZF-7 ) (brand is not critical)
High-frequency vibrating atomizer, 5 W, 5 V (Purchase from Alibaba, SKG, catalog number: 37288557693 )
Inverted microscope equipped with a 100 W halogen tungsten lamp, a multipurpose dry condenser and polarizer/analyzer combination (Nikon, model: Ellipse Ti-U )
Long working distance objective, CFI TU Plan FLUOR BD 20x, NA/WD 0.45/4.5 mm (Nikon)
20x objective, Plan Fluor 20x, NA/WD 0.5/ 2.1 mm (Nikon)
Upright metallographic microscope equipped with a 100 W halogen tungsten lamp, and an Epi illuminator containing an orthogonal polarizer/analyzer module (Nikon, model: LV100D )
High-precision piezo-Z positioning stage (PZ-2150)
Color CMOS camera (Olympus, model: DP74 )
Upright dark-field microscope with a 100 W halogen tungsten lamp and a dark-field oil condenser (Nikon, model: 80i )
Inverted phase contrast microscope (Olympus, model: CKX53 )
Software
ImageJ (An open source software written in Java, https://imagej.nih.gov/ij/)
Matlab (The MathWorks, Inc., https://www.mathworks.com/)
Multi-particle tracking software (self-developed in MATLAB, Github: https://github.com/threebullets/ParDet)
Origin 8 (OriginLab, https://www.originlab.com/)
PIVlab-particle image velocimetry (PIV) tool (William Thielicke, 2014) (GUI based tool developed by William Thielicke, https://pivlab.blogspot.com/)
SPSS (IBM, https://www.ibm.com/analytics/spss-statistics-software/)
Procedure
文章信息
版权信息
© 2020 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Feng, J. and He, Y. (2020). Long-distance Transport in Bacterial Swarms Revealed by Single Nanoparticle Tracking. Bio-protocol 10(21): e3812. DOI: 10.21769/BioProtoc.3812.
分类
分子生物学 > 纳米颗粒 > 传感器
微生物学 > 体内实验模型 > 细菌
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link













