- EN - English
- CN - 中文
An Operant Conditioning Model Combined with a Chemogenetic Approach to Study the Neurobiology of Food Addiction in Mice
操作式条件反射模型结合化学遗传学方法研究小鼠食物成瘾的神经生物学机制
发布: 2020年10月05日第10卷第19期 DOI: 10.21769/BioProtoc.3777 浏览次数: 5773
评审: Arnau Busquets-GarciaGian Marco LeggioMarco Venniro

相关实验方案

基于 rAAV-α-Syn 与 α-Syn 预成纤维共同构建的帕金森病一体化小鼠模型
Santhosh Kumar Subramanya [...] Poonam Thakur
2025年12月05日 1660 阅读
Abstract
The study of food addiction comprises 3 hallmarks that include the persistence to response without an outcome, the strong motivation for palatable food, and the loss of inhibitory control over food intake that leads to compulsive behavior in addicted individuals. The complex multifactorial nature of this disorder and the unknown neurobiological mechanistic correlation explains the lack of effective treatments. Our operant conditioning model allows deciphering why some individuals are vulnerable and develop food addiction while others are resilient and do not. It is a translational approach since it is based on the Diagnostic and Statistical Manual of Mental Disorders 5th edition (DSM-5) and the Yale Food Addiction Scale (YFAS 2.0). This model allows to evaluate the addiction criteria in 2 time-points at an early and a late period by grouping them into 1) persistence to response during a period of non-availability of food, 2) motivation for food with a progressive ratio, and 3) compulsivity when the reward is associated with a punishment such as an electric foot-shock. The advantage of this model is that it allows us to measure 4 phenotypic traits suggested as predisposing factors related to vulnerability to addiction. Also, it is possible to evaluate the long food addiction mouse model with mice genetically modified. Importantly, the novelty of this protocol is the adaptation of this food addiction model to a short protocol to evaluate genetic manipulations targeting specific brain circuitries by using a chemogenetic approach that could promote the rapid development of this addictive behavior. These adaptations lead to a short food addiction mouse protocol, in which mice follow the same behavioral procedure of the early period in the long food addiction protocol with some variations due to the surgical viral vector injection. To our knowledge, there is no paradigm in mice allowing us to study the combination of such a robust behavioral approach that allows uncovering the neurobiology of food addiction at the brain circuit level. We can study using this protocol if modifying the excitability of a specific brain network confers resilience or vulnerability to developing food addiction. Understanding these neurobiological mechanisms is expected to help to find novel and efficient interventions to battle food addiction.
Keywords: Food addiction (食物成瘾)Background
In the last years, food addiction has gained attention due to the increasing prevalence worldwide (19.9 %) and currently represents a high cost to the individual and the society without any effective treatment available (Pursey et al., 2014). The current diagnosis is performed by a recently validated tool, the Yale Food Addiction Scale 2.0 (YFAS 2.0). This instrument is based on the criteria applied in the 5th edition of the Statistical Manual of Mental Disorders (DSM-5) for substance use disorders, taking into account the increasing evidence suggesting that food addiction shares its neurobiological substrates with drug addiction (Lindgren et al., 2017). Food addiction is a complex multifactorial brain disorder resulting from the dynamic interaction among multiple gene networks and multiple environmental factors impacting brain development and function, leading to individual differences among the population (Hamer, 2002; Nestler et al., 2015). For this reason, not all individuals become addicted and extreme subpopulations can be distinguished with an addicted and non-addicted phenotype (Piazza and Deroche-Gamonet, 2013). Conversely, the precise neurobiological mechanisms underlying both phenotypes are still unclear despite the well-known common brain areas involved in addictive processes that include the basal ganglia, extended amygdala, and prefrontal cortex (Koob and Volkow, 2016; Moore et al., 2017). The current protocol improves previous studies because it has the inclusion of a short protocol for evaluating food addiction phenotype in genetically modified mice that present anticipation of food addiction development. In this protocol, the development of loss of control over food intake that characterizes addiction is revealed by measuring compulsivity, motivation, and persistence in different time-points. Compared to other operant models, this has the advantage of measuring other phenotypic traits such as impulsivity, cognitive flexibility, appetitive associative learning, and aversive conditioning. These traits are potential predictors of the development of food addiction. In this study, the main aim is to describe a replicable protocol that allows deciphering the neurobiological mechanisms involved in the resilient and vulnerable phenotypes to develop a food addiction. To address this major question, we describe a protocol with a reliable behavioral approach that can be adapted to combine a viral vector approach with chemogenetic manipulations. These findings will help to design new strategies to focus the strength in the prevention of the transition to food addiction by increasing the inhibitory control of individuals exposed to unhealthy environmental conditions.
Materials and Reagents
Materials
- Chocolate-flavored pellets (20 mg/pellet, 5TUL #1811223, TestDiet, Richmond, IN, USA)
- Microsyringe (10 μl, Model 1701 N S.Y.R., Cemented N.D.L., 26 ga, 2 in, point style 3, #80039, Hamilton company, N.V., U.S.A.)
- Polyethylene tubing (PE-20, #C315CT , Plastics One, U.K.)
- Bilateral guide cannula (26-gauge cannula cut 12 mm below pedestal, #C235GS-5/Spc, Plastics One, U.K.)
- Bilateral internal cannula (33-gauge internal cannula fits 12 mm C235GS-5/Spc with 3 mm projection, Plastics One, U.K.)
- Osmotic minipumps (flow rate of 0.25 μl/h for 28 days, Model 2004, #0000298 , Alzet, CA, U.S.A.)
- Scalpel ( #02-036-040 , AgnTho's, Sweden)
- Manual drill ( DH-1 , Plastics One, U.K.)
- Blunt-tipped surgical scissors ( #03-022-105 , AgnTho's, Sweden)
- Curved iris forceps ( #08-513-005 , AgnTho's, Sweden)
- Suture clips ( #08-922-125 , AgnTho's, Sweden)
- Surgical clips ( #22-620-007 , AgnTho's, Sweden)
- Suture thread (black braided silk, TB10, 3/8 TRIANG 15 mm 4/0 90 cm, #55327-50U , LorcaMarín, Spain)
Reagents
- Distilled water
- Ethanol 70%
- Iodine (Betadine, 500 ml, #716720 , MEDA Pharma S.A.U., Spain)
- Physiological saline (0.9%, 250 ml, #999790.8 , Laboratorios ERN, Spain)
- Glucose serum (GlucosaVet 5g/100ml, #1248 ESP , B. Braun Vet Care, Spain)
- Xilin night (5 g, #2919-PS-CM , Visufarma, Spain)
- Blastoestimulina (1%, 30g, # 719385 , Almirall, Spain)
- Vetflurane (Isoflurane, 250 ml, #2199-ESP , Virbac, Spain)
- Clozapine N-oxide (CNO, 25 mg, #BML-NS105-0025 , Enzo Life Sciences, NY) diluted in 0.9% sterile saline (5 mg/ml)
- Anesthesia reagents
- Ketamine hydrochloride (75 mg/kg of body weight, 10 ml, Ketamidor, #580393 , Richterpharma ag, Austria) dissolved in sterile 0.9% physiological saline
- Medetomidine hydrochloride (1 mg/kg of body weight, #570686 , Domtor; Esteve, Spain) dissolved in sterile 0.9% physiological saline
- Atipamezole hydrochloride (2.5 mg/kg of body weight, #570559 , Revertor; Virbac, Spain) dissolved in sterile 0.9% physiological saline
- Gentamicine (1 mg/kg of body weight, #999037 , Genta-Gobens; Laboratorios Normon, Spain) dissolved in sterile 0.9% physiological saline
- Meloxicam (2 mg/kg of body weight, Metacam; #059/02/08CVFPT , Boehringer Ingelheim, Rhein) dissolved in sterile 0.9% physiological saline
- Viral vectors (storage at -80 °C). Examples
- AAV8-hSyn-DIO-hM4D(Gi)-mCherry (1.21E + 13 gc/ml, Viral Vector Production Unit of Universitat Autònoma de Barcelona)
- AAV8-hSyn-DIO-mCherry (1.19E + 13 gc/ml, Viral Vector Production Unit of Universitat Autònoma de Barcelona)
- AAVrg pmSyn1-EBFP-Cre (6 x 1012 vg/ml, Addgene, viral prep # 51507-AAVrg )
Equipment
- Mouse operant self-administration chambers (Model ENV-307A-CT , Med Associates, Georgia, VT, U.S.A.)
The operant chambers are equipped with two retractable levers (#ENV-312-2M, Med Associates), one randomly selected as the active lever and the other as the inactive. Pressing on the active lever results in a food pellet delivery paired with a stimulus-light (associated-cue, #ENV-321M, Med Associates), located above the active lever, and while pressing on the inactive lever has no consequences. A food dispenser (#ENV-303M pellet receptacle, #ENV-203M-20, modular pellet dispenser, Med Associates) equidistant between the two levers permit the delivery of food pellets when required. The floor of the chambers is a grid floor (#ENV-307A-GF, Med Associates) that serves to deliver electric foot shocks in the session of shock test and serves as a contextual cue in the session of shock-induced suppression the day after the shock test. During the rest of the self-administration sessions, a metal sheet with holes is placed above the grid floor. Thus, mice can discriminate between different contexts. A house light is placed on the ceiling of the chamber (#ENV-315M, Med Associates). The chambers are made of aluminum and acrylic and are housed in sound- and light-attenuated boxes equipped with fans to provide ventilation and white noise. (Figure 1).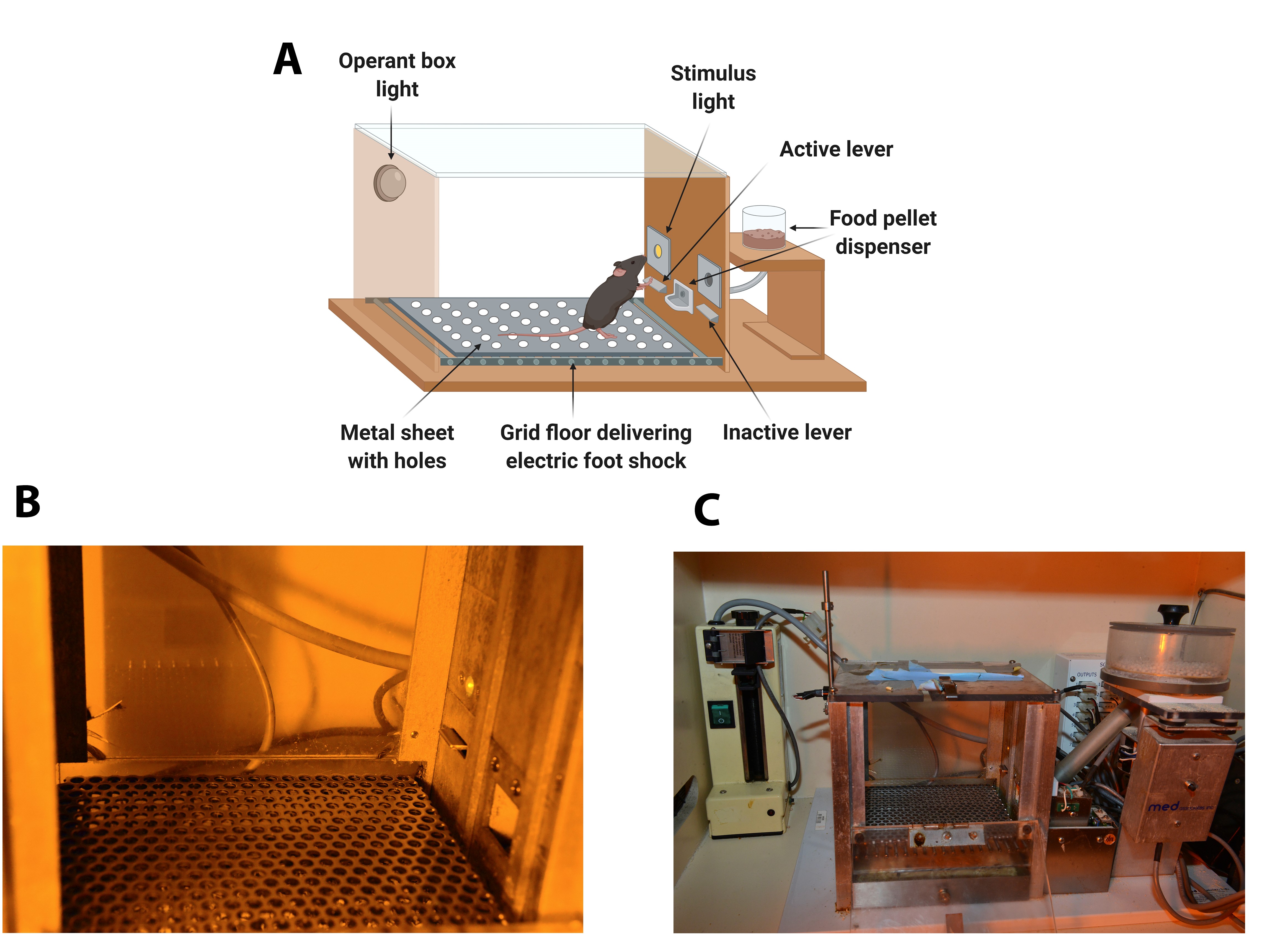
Figure 1. Diagram and images of an operant self-administration chamber. A. Diagram of the operant chamber. The operant chamber is equipped with two retractable levers (active and inactive), a stimulus-light, and a food pellet dispenser. The floor of the chamber is a grid floor that delivers electric foot shocks in the session of shock test but is covered during the rest of self-administration sessions with a metal sheet with holes. B. Image with detail of the panel containing the active lever signaled by the cue-light and the metal sheet with holes. C. General view of the Skinner Box with the operant box light, the active lever, the cue-light, the grid floor that delivers electric foot shocks, the metal sheet with holes, and the food dispenser. - Stereotaxic apparatus (100-micron resolution, Model 900 , Koft instruments, C. A., U.S.A.)
- Standing magnifier (OPMI 1 FR, Carl Zeiss, U.S. A.)
- Microinfusion pump (P.H.D. 2000, #MA1 70-20xx , Harvard Apparatus, Holliston, MA, U.S.A.)
- Animal trimmer ( #M630 , Artero, Spain)
- Cold light (Leica C.L.S. 150x, Leica Microsystems, Spain)
- Heating pad ( #N2P 220-230 , 60W, 50Hz, Daga, Spain)
- Hot bead sterilizer (FST 250, #18000-45 , AgnTho's, Sweden)
Software
- Med-PC Software (Med Associates Inc, U.S.A.). Software that registers all the behavior in the operant self-administration chambers
- GraphPad Prism software (GraphPad Software, U.S.A.) to perform all graphs
- SPSS software (I.B.M., version 25) to perform statistical data analysis
Procedure
文章信息
版权信息
© 2020 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Martín-García, E., Domingo-Rodriguez, L. and Maldonado, R. (2020). An Operant Conditioning Model Combined with a Chemogenetic Approach to Study the Neurobiology of Food Addiction in Mice. Bio-protocol 10(19): e3777. DOI: 10.21769/BioProtoc.3777.
分类
神经科学 > 神经系统疾病 > 动物模型
神经科学 > 行为神经科学 > 实验动物模型
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link










