- EN - English
- CN - 中文
Determination of Intracellular Ca2+ Concentration in the Human Pathogens Trypanosomatids Leishmania mexicana and Trypanosoma cruzi by the Use of the Fluorescent Ca2+ Indicator Fura-2
利用荧光Ca2+指示剂Fura-2测定人类病原体锥虫,墨西哥利什曼原虫和克氏锥虫中细胞内Ca2+的浓度
(*contributed equally to this work) 发布: 2020年09月20日第10卷第18期 DOI: 10.21769/BioProtoc.3766 浏览次数: 3759
评审: Alexandros AlexandratosEvangelia XingiAnonymous reviewer(s)

相关实验方案
用于研究肺部感染与免疫反应的离体肺组织培养模型,以 SARS-CoV-2 为 RNA 病毒研究示例
Elena V. Maryukhnich [...] Elena J. Vasilieva
2025年12月20日 710 阅读
Abstract
Ca2+ is an essential signaling messenger in all eukariotic cells, playing a pivotal role in many cellular functions as cell growth control (differentiation, fertilization and apoptosis), secretion, gene expression, enzyme regulation, among many others. This basic premise includes trypanosomatids as Trypanosoma cruzi and various species of Leishmania, the causative agents of Chagas disease and leishmaniasis respectively, where intracellular Ca2+ concentration ([Ca2+]i) has been demonstrated to be finely regulated. Nevertheless [Ca2+]i has been difficult to measure because of its very low cytoplasmic concentration (typically around 50-100 nM), when compared to the large concentration in the outside milieu (around 2 mM in blood). The development of intracellular fluorescent Ca2+-sensitive indicators has been of paramount importance to achieve this goal. The success was based on the synthesis of acetoximethylated derivative precursors, which allow the fluorescent molecules typically composed of many hydrophilic carboxyl groups responsible for its high affinity Ca2+-binding (and therefore very hydrophilic), to easily cross the plasma membrane. Once in the cell interior, unspecific esterases split the hydrophobic moiety from the fluorescent backbone structure, releasing the carboxyl groups, transforming it in turn to the acid form of the molecule, which remain trapped in the cytoplasm and regain its ability to fluoresce in a Ca2+-dependent manner. Among them, Fura-2 is by far the most used, because it is a ratiometric (two different wavelength excitation and one emission) Ca2+ indicator with a Ca2+ affinity compatible with the [Ca2+]i. This protocol essentially consists in loading exponential phase parasites with Fura-2 and recording changes in [Ca2+]i by mean of a double wavelength spectrofluorometer. This technique allows the acquisition of valuable information about [Ca2+]i changes in real time, as a consequence of diverse stimuli or changes in conditions, as addition of drugs or different natural modulators.
Keywords: Intracellular Ca2+ measurements (细胞内Ca2+测定)Background
Aiming to develop new drugs against infections caused by different trypanosomatids, efforts have been invested in the elucidation of the physiological mechanism responsible for intracellular ionic homeostasis, in particular Ca2+ ions, which are known to play a pivotal role as a second messenger in Trypanosoma cruzi and Leishmania spp.The intracellular Ca2+ concentration ([Ca2+]i) is finely regulated (Benaim and García., 2011; Benaim et al, 2020) in these kinetoplastids. Leishmaniases are vector-borne parasitic neglected diseases caused by at least 20 species of the genus Leishmania, and are transmitted between mammalian hosts by female sandflies (Burzaet al., 2018). Chagas disease is another particularly neglected human infection, caused by Trypanosoma cruzi, transmitted by triatomine bugs when infectious parasites in the feces of the vector enter in the skin of the mammalian host at the bite site where scratching is provoked (Santos et al., 2020).
Prior to Fura-2, the most popular method for measuring [Ca2+]i in cells was to monitor the fluorescence of an indicator called Quin-2. Nevertheless this fluorophore has severe limitations due to its short excitation wavelength, non-ratiometric nature and low quantum yield (Grynkiewicz et al., 1985). Also, Quin-2 produces the quenching of large amounts of intracellular Ca2+ that could strongly interfere with the minute changes in the [Ca2+]i that are the focus of this protocol. For this reason, Quin-2 has been used instead as an intracellular Ca2+ chelator, when information under this extreme cellular condition is required. However, BAPTA is most preferred for this use because even though it has a similar molecular structure and a comparable affinity for Ca2+ as does Quin 2, BAPTA has a very weak fluorescence emission, thus having less interference with the [Ca2+]i measurement (Moreno et al, 1994). Fura-2 indeed overcomes all these limitations. The main advantage of using ratiometric dyes like Fura-2, when compared to single wavelength probes, is that the ratio signal is independent of the dye concentration, illumination intensity, and optical path length, allowing thereby to accurately determine the concentration of intracellular calcium (Barreto-Chang and Dolmetsch, 2009). In cell suspensions, accurate determinations of [Ca2+]i changes can be made by the use of Fura-2, especially in combination with various available pharmacological agents (Patel et al., 2012).
The simple and relatively fast protocol presented here has been widely used in the search and evaluation of several potential chemotherapeutic anti-parasitic drugs to elucidate their mechanism of action against T. cruzi and several Leishmania spp. For example, one can determine the source of Ca2+ (intracellular vs. extracellular) in a particular rise of the [Ca2+]i observed in a particular condition, for example a treatment with a drug, in order to verify if the compound acts at the plasma membrane level (channels, receptors), at intracellular compartments (mitochondria, endoplasmic reticulum, acidocalcisomes) or at both (Benaim et al., 2020). This is simply achieved by performing the same experiment in the presence or absence (using EGTA, a Ca2+ chelating agent) of extracellular Ca2+.
A typical example of the Fura-2 use in Trypanosoma cruzi is depicted in Figure 1. In A (blue trace) it can be seen that addition of sphingosine (Sph) to epimastigotes loaded with Fura 2 induces an increase in [Ca2+]i, which is specific for this particular sphingolipid, since addition of Sphingosine-1-Phosphate (Sph-1-P), Ceramide-1-Phosphate (Cer-1-P) or Ceramide (Cer) were without any discernible effect. On the other hand, when EGTA was added to study the effect of Sph in the absence of extracellular calcium (red trace), the sphingolipid, instead of inducing an increase in the [Ca2+]i, caused a small decrease.This could represent a release of the low cytoplasmic Ca2+ to the extracellular space passing by the channel, when itencountered the chelator EGTA. In Figure1B are depicted the effects of nifedipine, a classical human L Type VGCC antagonist on the parasite channel. The green trace shows that nifedipine fully blocks the action of Sph added (indicated by a green arrow). As it is observed (Black trace) the miltefosine, is also able to open this channel, mimicking its effect in L. mexicana (Benaim et al., 2013). Miltefosine is the only oral drug approved for the treatment of visceral leishmaniasis and is also effective against T. cruzi. But, differently to the effect of the natural sphingolipid, nifedipine was not able to totally block the action of this drug (Blue trace), suggesting that these compounds act via a different mechanism on the Ca2+ channel.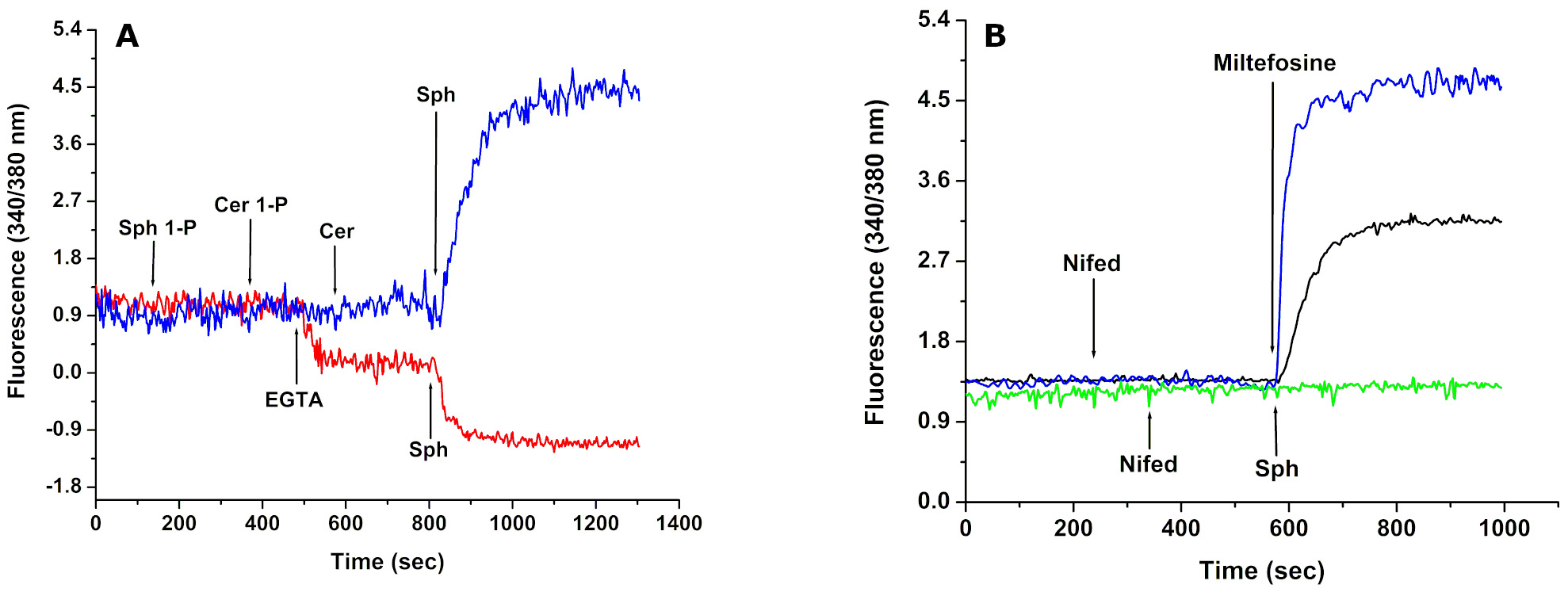
Figure 1. Effect of Sphingosine-1-P (Sph-1-P), Ceramide -1-P (Cer-1-P),Ceramide (Cer) and Sphingosine (Sph), Miltefosine and Nifedipine (a specific L-type VGCC channel blocker) on the intracellular Ca2+ concentration of Trypanosoma cruzi. A. Sph-1-P, Cer-1-P, Cer and Sph were added sequentially to a final concentration of 10 µM in the presence of 2 mM CaCl2 (blue trace) and in the absence of extracellular Ca2+ (red trace). B. Sphingosine (10 µM, arrow) was added after addition of nifedipine (4 µM, arrow) in the presence of extracellular Ca2+ (green trace). Miltefosine (4 µM) was added in the presence of extracellular CaCl2 (blue trace). Nifedipine (40 µM, arrow) was added before the addition of Miltefosine (4 µM) in the presence of extracellular Ca2+ (black trace). (see text for details). Taken from Rodriguez-Duran et al. (2019) FEBS J, 286, 3909-3925. Copyright 2019. FEBS PRESS.
Finally, we used as example the effect of a potent anti-tuberculosis drug known to affect T. cruzi, L. mexicana (Benaim et al., 2020) and L. donovani (Gil et al., 2020) which instead of acting through the plasma membrane Ca2+ channels as miltefosine does, it exerts its action on intracellular organelles, like the parasites’ mitochondrion and/or acidocalcisomes. Thus, it can be observed in Figure 2, that the effect of the drug is the same in the presence (A) or in the absence (B) of extracellular Ca2+, since the cation is released from the mentioned intracellular organelles.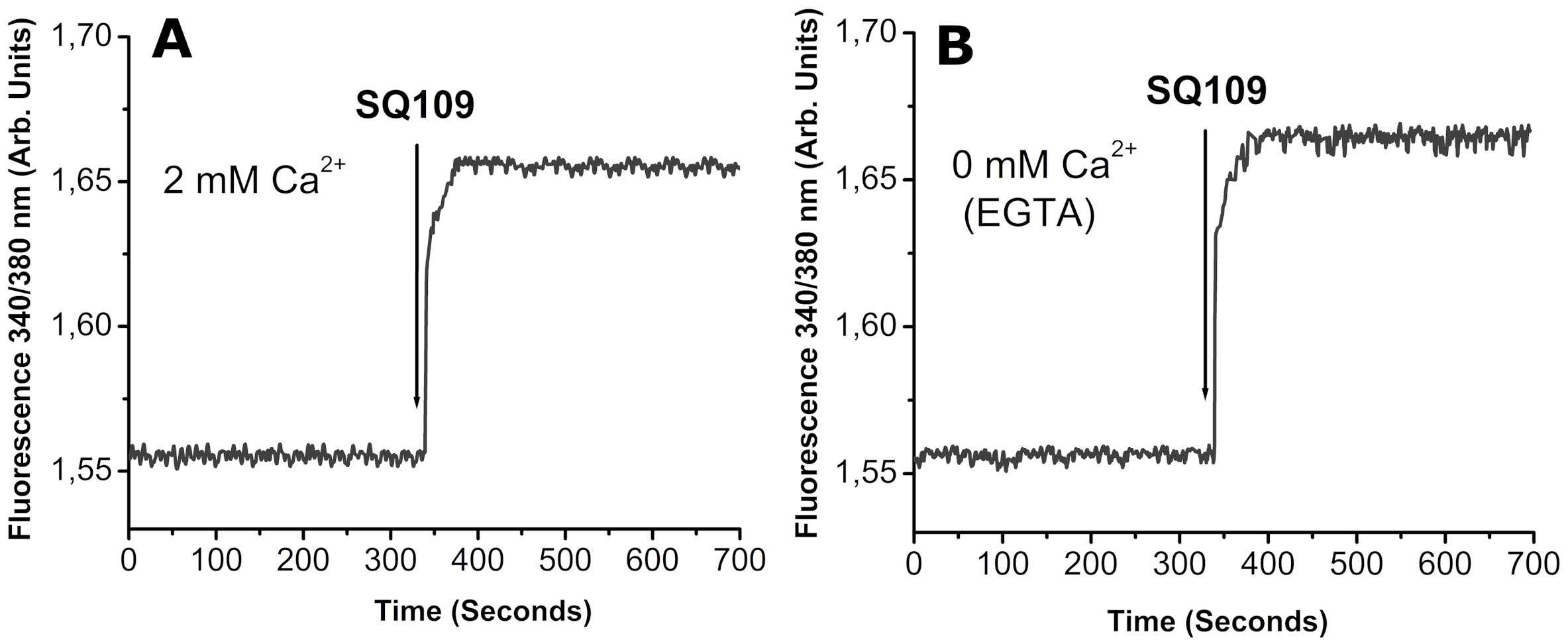
Figure 2. Effects of the antituberculosis drug SQ109 on Ca2+ fluxes in Leishmania mexicana. (A) Intracellular Ca2+ concentration in the presence of 2 mM external Ca2+ and (B) Same as panel A but in the absence of external Ca2+ (2 mM EGTA). Taken from García-García et al. (2016) Antimicrob Agents Chemother. 60: 6386-6389, Copyright 2016. American Chemical Society.
Materials and Reagents
- Pluronic acid F-127 (Thermo Fisher Scientific, InvitrogenTM, catalog number: P3000MP )
20% (w/v) stock in DMSO. An amphiphilic mild detergent that facilitates Fura-2 AM solubilization - Probenecid (Thermo Fisher Scientific, InvitrogenTM, catalog number: P36400 )
An inhibitor of the anionic transporters improving intracellular accumulation of the fluorophore - Fura-2 acetoxymethyl ester (Fura-2 AM) (Thermo Fisher Scientific, InvitrogenTM, catalog number: F1225 )
- Digitonin (Merck, Sigma-AldrichTM, catalog number: 300410 )
- EGTA (Ethylene-bis(oxyethylenenitrilo)tetraacetic acid) (Merck, Sigma-AldrichTM, catalog number: E3889 )
- Calcium chloride (CaCl2) (Merck, Sigma-AldrichTM, catalog number: C1016 )
- Potassium chloride (KCl) (Merck, Sigma-AldrichTM, catalog number: P9333 )
- Potassium phosphate monobasic (KH2PO4) (Merck, Sigma-AldrichTM, catalog number: P0662 )
- Sodium phosphate dibasic (Na2HPO4) (Merck, Sigma-AldrichTM, catalog number: S0876 )
- Magnesium sulfate (MgSO4) (Merck, Sigma-AldrichTM, catalog number: M7506 )
- Sodium chloride (NaCl) (Merck, Sigma-AldrichTM, catalog number: S7653 )
- HEPES (Merck, Sigma-AldrichTM, catalog number: H0887 )
- Glucose (Merck, Sigma-AldrichTM, catalog number: G8270 )
- Modified Tyrode’s loading buffer (MTLB) (pH 7.4) (see Recipes)
- MTLB without Ca2+(pH 7.4) (see Recipes)
Equipment
- Neubauer chamber
- Spectrofluorometer with Fast Filter Accessory (PerkinElmer, model: LS-55 ) or Spectrofluorometer HITACHI DW 7000
- Benchtop Centrifuge (Eppendorf, model: 5415D )
- Quartz SUPRASIL Macro/Semi-micro Cell (PerkinElmer, catalog number: B0631132 )
Software
- FL WinLab (PerkinElmer)
- Excel® (Microsoft)
- OriginPro 6 (OriginLab Corporation, https://www.originlab.com/)
Procedure
文章信息
版权信息
© 2020 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Rey-Cibati, A., Valladares-Delgado, M. and Benaim, G. (2020). Determination of Intracellular Ca2+ Concentration in the Human Pathogens Trypanosomatids Leishmania mexicana and Trypanosoma cruzi by the Use of the Fluorescent Ca2+ Indicator Fura-2. Bio-protocol 10(18): e3766. DOI: 10.21769/BioProtoc.3766.
分类
微生物学 > 微生物-宿主相互作用 > 离体模型
细胞生物学 > 基于细胞的分析方法 > 钙离子稳态
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link