- EN - English
- CN - 中文
In vitro Crosslinking Reactions and Substrate Incorporation Assays for The Identification of Transglutaminase-2 Protein Substrates
转谷氨酰胺酶-2蛋白底物的体外交联反应和底物掺入鉴定
发布: 2020年06月20日第10卷第12期 DOI: 10.21769/BioProtoc.3657 浏览次数: 4219
评审: Raquel Brandao HagaAleksei A TikhonovKossay Zaoui

相关实验方案
一种改良的酰基-RAC方法分离视网膜棕榈酰蛋白质组,并通过LC-MS/MS进行后续检测
Sree I. Motipally [...] Saravanan Kolandaivelu
2023年04月20日 2617 阅读
Abstract
Transglutaminase (TG2) catalyzes protein crosslinking between glutamyl and lysyl residues. Catalytic activity occurs via a transamidation mechanism resulting in the formation of isopeptide bonds. Since TG2-mediated transamidation is of mechanistic importance for a number of biological processes, assays that enable rapid and efficient identification and characterization of candidate substrates are an important first-step to uncovering the function of crosslinked proteins. Herein we describe an optimized and flexible protocol for in vitro TG2 crosslink reactions and substrate incorporation assays. We have previously employed these techniques in the identification of the protein high mobility group box 1 (HMGB1) as a TG2 substrate. However, the protocol can be adapted for identification of any candidate transamidation substrate.
Keywords: Transglutaminase (谷氨酰胺转移酶)Background
Transglutaminase (TG2) is a member of the transglutaminase enzyme family that catalyzes calcium-dependent transamidation reactions. The transglutaminase enzyme family includes TG1-7 and FXIIIa, as well as band 4.2, a non-catalytically active member that plays a role in protein scaffolding (Satchwell et al., 2009; Gundemir et al., 2012). TG2 is unique to the family in that it is ubiquitously expressed and pleiotropic in function; in addition to protein crosslinking TG2 functions as a protein disulfide isomerase (Hasegawa et al., 2003; Mastroberardino et al., 2006), G-protein (Nakaoka et al., 1994; Baek et al., 1996; Vezza et al., 1999), and kinase (Mishra and Murphy, 2004; Mishra et al., 2006 and 2007).
Protein crosslinking occurs between the γ-carboxamide group of a peptide-bound glutamyl residue (a glutamine donor substrate) and the ϵ-amino group of a lysyl residue (lysine acceptor substrate), resulting in the formation of ϵ-(γ-glutamyl) lysine isopeptide bonds (Folk and Finlayson, 1977). A schematic of the crosslink reaction is shown in Figure 1.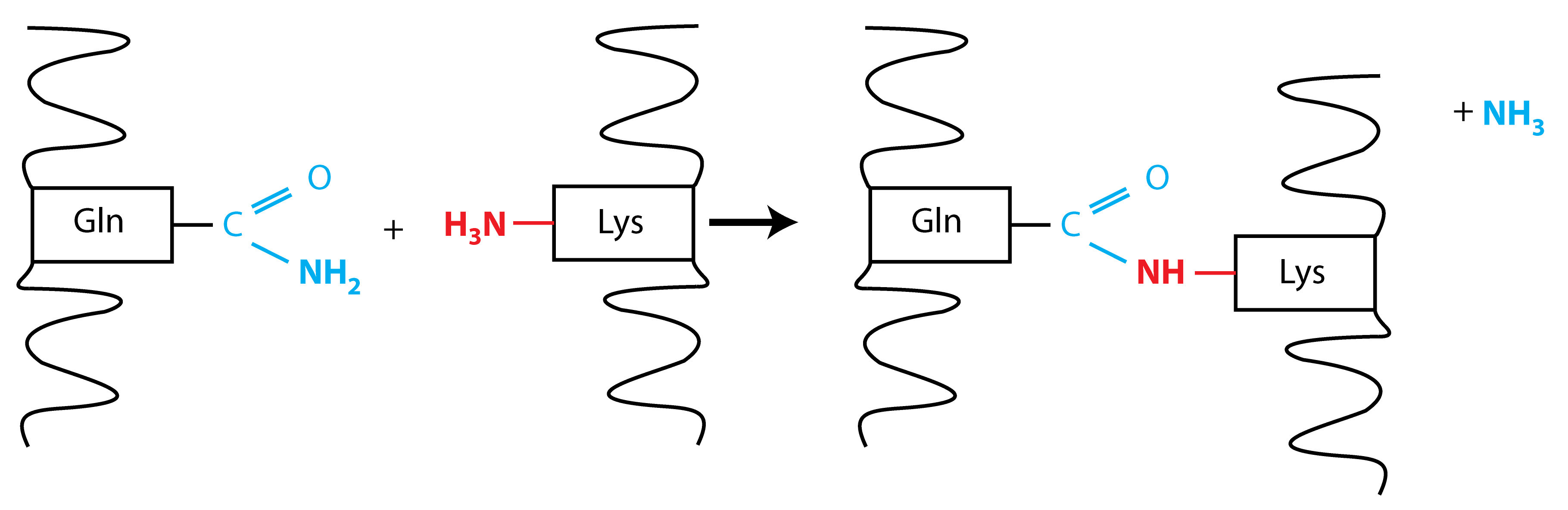
Figure 1. Schematic for the TG2 protein crosslink reaction. Protein crosslinking occurs by a transamidation reaction between the γ-carboxamide group of a peptide-bound glutamyl residue (shown in blue) and the ϵ-amino group of a lysyl residue (shown in red). Glutamine loses its amino group during the reaction, resulting in the formation of an ϵ-(γ-glutamyl) lysine isopeptide bond.
Although Tris-based buffers have been traditionally used for in vitro TG2 crosslink reactions, we found these buffers to be problematic for certain substrates, particularly those containing lysine donors only. Since Tris is a primary amine, it can potentially function as an acyl acceptor substrate in the transamidation reaction, competing with peptide-bound lysyl residues on protein substrates. To circumvent issues with Tris-based buffers, we instead used MOPS based buffer system for crosslink reactions. Since MOPSs is a tertiary amine, this avoids any potential issues with Tris- inhibition of the enzymatic reaction.
After confirmation that a protein is crosslinked by TG2, delineation of glutamyl vs. lysyl crosslink sites in the protein is an important next step to determine whether lysine and/or glutamine residues participate in the transamidation reaction.
The general scheme for in vitro crosslink reactions and substrate incorporation assays is described as follows:
1) Determine whether or not the protein of interest is a TG2 transamidation substrate.
Crosslink reactions between TG2 and purified recombinant forms of the putative substrate are performed in vitro and followed by Western blot analysis. This reaction can also be conducted in the context of endogenous proteins by using as substrates lysates from cell or tissue sources that contain the protein of interest instead of recombinant proteins. In either case, the appearance of a slower-migrating, size-shifted band by Western blot using antibodies against the putative substrate indicates a positive result. Regardless of whether purified recombinant proteins or endogenous sources from a cell or tissue lysate are included in the crosslink reaction, it is important to include the appropriate controls. The critical controls include reactions without TG2 enzyme, calcium, and/or using mutant TG2 enzyme lacking transamidation activity are for non-ambiguous data interpretation. Typical Western blot results for an in vitro crosslink reaction using purified recombinant HMGB1 are shown in Figure 2. Additionally, blocking peptide assays with the peptide antigen used to generate the antibody can be utilized to compete away the signals, confirming antibody specificity for TG2-modified substrates (Willis et al., 2018, Figure 3G).
2) Characterization of transamidation donor sites by substrate incorporation assays.
To determine whether the substrate in question undergoes crosslinking via glutamine or lysine donor residues or both, crosslink reactions between the purified recombinant proteins are supplemented with biotin-labeled glutamine- or lysine-donor probes. The crosslink reactions are then separated on SDS-PAGE and analyzed for biotin signal by Western blot with streptavidin-conjugated HRP. The utility of performing the substrate incorporation assay in this manner is that (in contrast to an ELISA-plate based format), additional qualitative information is gleaned about the size and relative abundance of probe-labeled reaction products on the gel. Additionally, specific bands of interest can be excised from the gel for protein identification or crosslink mapping by mass spectrometry (Willis et al., 2018).
Materials and Reagents
- 10 cm tissue culture plates (Corning, Catalog number: 430293 )
- 1.5 ml microcentrifuge tubes (USA Scientific, catalog number: 1615-5510 )
- 15 ml conical tubes (Thermo Scientific, catalog number: 339651 )
- Transglutaminase from guinea pig liver (Sigma-Aldrich, catalog number: T5398 )
- MOPS [3-(N-morpholino)propanesulfonic acid] (Sigma-Aldrich, catalog number: M1254 )
- Biotin-pentylamine (EZ-LinkTM Pentylamine-Biotin) (Thermo Scientific, catalog number: 21345 )
- Biotin-TVQQEL (a.k.a. A25 peptide) (Zedira, catalog number: B001 )
- Roche Complete protease inhibitor cocktail (Sigma, catalog number: 11697498001 )
- Dithiothreitol (DTT) (Sigma, catalog number: D9163 )
- PMSF (phenylmethanesulfonyl fluoride) (Sigma, catalog number: 78830 )
- Western blotting materials
- 4-12% polyacrylamide gels (Thermo Scientific catalog number: NW04120BOX )
- Mini gel tank (Thermo Scientific catalog number: A25977 )
- Nitrocellulose or PVDF membranes
- Electrophoretic transfer apparatus (GE Biosciences TE-22 )
- Tris [tris(hydroxymethyl)aminomethane] base (Fisher, catalog number: BP152-500 )
- HMGB1(high mobility group box protein 1; Genscript, catalog number: Z02803 )
- PBS (phosphate-buffered saline) (Thermo Scientific, catalog number: 10010049 )
- NaCl (Sigma-Aldrich, catalog number: S7653 )
- Ethylenediaminetetraacetic acid (EDTA) (Sigma, catalog number: E9884 )
- Sodium deoxycholate (Sigma, catalog number: D6750 )
- Sodium dodecyl sulfate (SDS) (Sigma, catalog number: L3771 )
- NP-40 (a.k.a. Igepal CA-630) (Sigma, catalog number: I8896 )
- Glycerol (Sigma, catalog number: G6279 )
- Streptavidin-Horse-radish peroxidase (Streptavidin-HRP)
- Protease inhibitor 25x stock solution (see Recipes)
- Dithiothreitol (DTT) 1 M stock solution (see Recipes)
- Crosslink reaction buffer (see Recipes)
- TG2 reconstitution buffer (see Recipes)
- Modified RIPA buffer (see Recipes)
- 6x SDS-loading buffer (see Recipes)
- Tris/SDS pH 6.8 (see Recipes)
Equipment
- Incubator or heating block (Fisher Scientific, catalog number: 88-860-022 )
- Electrophoretic transfer apparatus (GE Healthcare, catalog number: TE-22 )
- Refrigerated benchtop centrifuge (Eppendorf, model: 5424R )
- Gel box for running gels (mini gel tank) (Thermo Scientific, catalog number: A25977 )
- Platform shaker (VWR, catalog number: 10127-876 )
- Tissue culture incubator (Thermo Scientific, model: Steri-CycleTM i160)
- Western blot imaging equipment (film + developer machine, Chemi-Doc, Licor, etc.)
Procedure
文章信息
版权信息
© 2020 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Willis, W. L., Foster, A., Henry, C., Wu, L. C. and Jarjour, W. (2020). In vitro Crosslinking Reactions and Substrate Incorporation Assays for The Identification of Transglutaminase-2 Protein Substrates. Bio-protocol 10(12): e3657. DOI: 10.21769/BioProtoc.3657.
- Willis, W. L., Wang, L., Wada, T. T., Gardner, M., Abdouni, O., Hampton, J., Valiente, G., Young, N., Ardoin, S., Agarwal, S., Freitas, M. A., Wu, L. C. and Jarjour, W. N. (2018). The proinflammatory protein HMGB1 is a substrate of transglutaminase-2 and forms high-molecular weight complexes with autoantigens. J Biol Chem 293(22): 8394-8409.
分类
生物化学 > 蛋白质 > 翻译后修饰
细胞生物学 > 基于细胞的分析方法 > 酶学测定
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link