- 提交稿件
- 订阅
- CN
- EN - English
- CN - 中文
- EN - English
- CN - 中文
Differential Fractionation of Erythrocytes Infected by Plasmodium berghei
感染伯氏疟原虫红细胞的差异分离
发布: 2020年06月05日第10卷第11期 DOI: 10.21769/BioProtoc.3647 浏览次数: 3537
评审: Alexandros AlexandratosDhiman Sankar PalKathrin Sutter

相关实验方案
通过制备连续聚丙烯酰胺凝胶电泳和凝胶酶谱分析法纯化来自梭状龋齿螺旋体的天然Dentilisin复合物及其功能分析
Pachiyappan Kamarajan [...] Yvonne L. Kapila
2024年04月05日 1192 阅读
Abstract
The study of host/pathogen interactions at the cellular level during Plasmodium intra-erythrocytic cycle requires differential extraction techniques aiming to analyze the different compartments of the infected cell. Various protocols have been proposed in the literature to study specific compartments and/or membranes in the infected erythrocyte. The task remains delicate despite the use of enzymes or detergents theoretically capable of degrading specific membranes inside the infected cell.
The remit of this protocol is to propose a method to isolate the erythrocyte cytosol and ghosts from the other compartments of the infected cell via a percoll gradient. Also, the lysis of the erythrocyte membrane is done using equinatoxin II, which has proven to be more effective at erythrocyte lysis regardless of the cell infection status, compared to the commonly used streptolysin. The parasitophorous vacuole (PV) content is collected after saponin lysis, before recovering membrane and parasite cytosol proteins by Triton X-100 lysis. The lysates thus obtained are analyzed by Western blot to assess the accuracy of the various extraction steps. This protocol allows the separation of the host compartment from the parasite compartments (PV and parasite), leading to potential studies of host proteins as well as parasite proteins exported to the host cell.
Background
The protocol presented herein is an improvement of a protocol that we initially published in Scientific Reports. The initial goal of this protocol was to perform the characterization of a P. berghei protein exported by the parasite to its host cell. This allowed the confirmation of immunofluorescence assays showing the export of the protein–and its blockage by Brefeldin A–and the analysis of its interactome upon export in the infected erythrocyte (Gnangnon et al., 2019).
With the aim to provide an improved protocol herein, we refined the technique reported previously by including a percoll gradient step to separate the host cell materials from the parasite ones. After enriching the infected erythrocytes via a Nycodenz® layer, we use Equinatoxin II (EQT–Anderluh et al., 1996) to lyse infected erythrocytes, instead of Streptolysin (SLO) as proposed previously (Nyalwidhe and Lingelbach, 2006; Heiber and Spielmann, 2014; Külzer et al., 2015). We made this choice since a few studies have shown that EQT lyses all erythrocytes independently from their infection status, whereas SLO lyses preferentially uninfected erythrocytes (Jackson et al., 2007; Schön et al., 2008). A percoll gradient is then used to separate the erythrocyte components from the parasite ones. The preparation of this gradient is performed similarly to the one used for P. falciparum (Heiber and Spielmann, 2014).
The parasites embedded in their parasitophorous vacuole (PV) are further treated using saponin, which specifically lyses the PV membrane (Külzer et al., 2015). The final lysis step involves Triton X-100 to recover membrane and parasite cytosol proteins (Heiber and Spielmann, 2014).
Other intermediate lysis steps can also be set up to analyze the parasite compartments more in depth (Ghosh et al., 2017). The different lysates are analyzed by Western blot. The choice of the antibodies used to detect protein markers of each purified compartment was based on previous studies and on antisera availability from different laboratories (see Table 1) (Knapp et al., 1990; Kina et al., 2000; Blisnick et al., 2006; Hliscs et al., 2013; Külzer et al., 2015; Meibalan et al., 2015; Ghosh et al., 2017). The techniques used here are optimized to the study of P. berghei–infected erythrocytes (the antibodies listed in Table 1 cross-react with P. berghei antigens despite being initially raised against proteins from other Plasmodium species) and may require some modifications when studying other Plasmodium species. In the case of P. falciparum, the reader can refer to suggestions made by Külzer et al. (2015).
The protocol presented here will enable the analysis of the membrane and cytosol of erythrocytes infected by Plasmodium (and maybe other erythrocyte-infecting pathogens like Babesia, following protocol optimization). Further refinements could allow a more precise and specific analysis of all the different compartments of the infected cell at once, which will favor the study of host/pathogen interactions over the parasite intra-erythrocytic development (see the “Notes” section at the very end of this manuscript). This is of critical importance since this phase of the development cycle of the parasite is responsible for the symptoms of malaria.
Materials and Reagents
Note: All chemicals are stored in dedicated rooms and safety cabinets. They are manipulated in accordance with product safety data sheets (SDS) and manufacturer instructions were followed for shelf-life (see below for storage temperatures–RT = Room Temperature).
Consumables:
- 1 ml syringes (Terumo, catalog number: SS+01T1 )
- 26 G ½” needles (Terumo, catalog number: AN-2613R1 )
- Transfer pipettes (rod volume 3 ml) (Biosigma, catalog number: 390514 )
- Microcentrifuge tubes (1.5 ml SafeLock) (Eppendorf, catalog number: 7953691744 )
- Polypropylene 15 ml centrifugation tubes (Sarstedt, catalog number: 62.554.502 )
- Parasites and Mice
- Plasmodium berghei ANKA parasites (kind gift from Dr. O. Silvie, CIMI, Paris, stored in liquid nitrogen, diluted in room temperature RPMI post-thawing prior to being i.p.-injected to mice)
- CD-1 mice (25-30 g male CD-1® IGS, Charles River)
Mice are i.p. infected with 106 infected red blood cells and parasitemia is monitored using thin blood smears. For the procedure detailed below, parasitemia of infected mice should be between 5% and 10%. This is usually obtained between day 5 and 10 post infection. - Sodic Heparin (25,000 UI/5 ml, Sanofi, stored at RT)
- iRBC (infected Red Blood Cell) sequential lysis
- Tris Base (Euromedex, catalog number: 200923-A , stored at RT)
- KCl (Sigma-Aldrich, catalog number: 60128 , stored at RT)
- CaNa2·EDTA (Sigma-Aldrich, catalog number: ED2SC , stored at RT)
- NaCl (Euromedex, catalog number: 1112 , stored at RT)
- MgSO4 (Sigma-Aldrich, catalog number: M7506 , stored at RT)
- Triton X-100 (Euromedex, catalog number: EU2000-B , stored at RT)
- D-Sorbitol (Sigma-Aldrich, catalog number: S1876 , stored at RT)
- Nycodenz® powder (Eurobio Ingen, catalog number: 1002424 , stored at RT)
- 10x PBS (Euromedex, catalog number: ET330-A , stored at RT)
- cOmpleteTM EDTA-free anti-protease cocktail (Roche, catalog number: 5056489001 , stored at 4 °C)
- Percoll solution (GE Healthcare, catalog number: 17-0891-01 , stored at 4 °C)
- RPMI medium 1640 w/HEPES (Thermo Fisher Scientific, catalog number: 12017599 , stored at 4 °C)
- Equinatoxin II enzyme (kind gift from Prof.Gregor Anderluh, National Institute of Chemistry, Ljubljana, Slovenia, aliquots are stored in PBS at -80 °C)
- Saponin lysis solution (Sigma Aldrich, catalog number: S7900 , powder stored at RT)
- 60% Nycodenz solution (see Recipes for storage conditions)
Nycodenz buffered medium
Nycodenz stock solution
60% Nycodenz solution (freshly prepared) - Equinatoxin II (EQT) lysis buffer (see Recipes)
- Saponin lysis solution (see Recipes)
Saponin 10x stock solution
1x Saponin lysis solution (freshly prepared) - Triton lysis buffer (see Recipes)
- Percoll gradient layers (see Recipes)
90% Percoll stock solution
80% Percoll layer
70% Percoll layer
60% Percoll layer
40% Percoll layer
- SDS-Page and Western blot analysis
- Tris Base (Euromedex, catalog number: 200923-A , stored at RT)
- SDS 20% solution (Sigma-Aldrich, catalog number: 0 5030 , stored at RT)
- Glycine (Euromedex, catalog number: 26-128-6405 , stored at RT)
- Glycerol (Euromedex, catalog number: EU3550 , stored at RT)
- Tween-20 (Sigma-Aldrich, catalog number: 8221840500 , stored at RT)
- Polyacrylamide gels (Bio-Rad Mini-PROTEAN® TGXTM Precast Gels, catalog number: 4561043 , stored at 4 °C
- PageRuler Prestained Protein Ladder (Thermo Scientific, catalog number: 26619 , stored at -20 °C)
- Low fat milk powder (Régilait, skimmed milk 0.8% fat, stored at RT)
- Western blot detection kit (SuperSignalTM West Dura Extended Duration Substrate, Thermo Fisher Scientific, catalog number: 34075 , stored at 4 °C)
- Antibodies (primary and secondary antibodies are listed in Table 1)
- 3x Sample buffer (see Recipes)
- Running buffer (see Recipes)
- Transfer buffer (see Recipes)
- Blocking solution (see Recipes)
- Western blot washing buffer (see Recipes)
Table 1. List of primary and secondary antibodies used in this study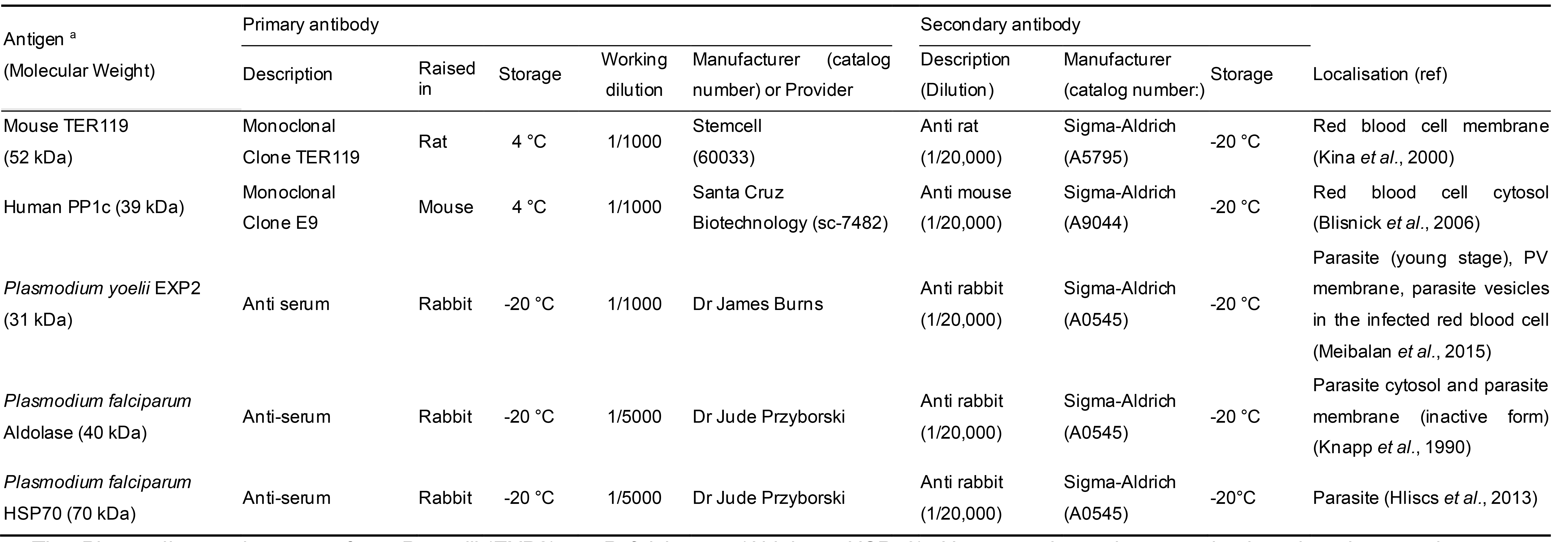
aThe Plasmodium antigens are from P. yoelii (EXP2), or P. falciparum (Aldolase, HSP70). However, the antiserum raised against these antigens cross react with P. berghei EXP2, Aldolase and HSP70 respectively. The antibody targeting human PP1c also recognizes mouse PP1c.
Equipment
- Laboratory rotating wheel (Stuart, model: SB3 )
- 50 ml Nalgene Oak Ridge High-Speed Centrifuge Tubes with sealing cap, PSF (Thermo Fisher Scientific, catalog number: 3137-0050 )
- Refrigerated centrifuge for microtubes (Eppendorf, model: 5427R )
- Centrifuge for 15 ml tubes (Eppendorf, model: 5810R )
- Superspeed centrifuge (Sorvall Lynx 4000 centrifuge with fixed angle rotor F20-12x50 LEX, ThermoScientific)
- Sonication device (Bioruptor plus, Diagenode, catalog number: B01020001 )
- Block heater (Stuart, model: SBH200D )
- Gel electrophoresis and transfer chamber (Bio-Rad Miniprotean Tetra Cell System)
- Filter paper for Western blot transfer (Whatman 3MM CHR, GE Healthcare, catalog number 3030-917 )
- Nitrocellulose membrane (Amersham protran Premium 0.45 Nitrocellulose, Thermo Fisher Scientific, catalog number: 15269804 )
- Vacuum sealer (KitchenGynti)
- Rocker platform (Labnet reciprocal, 3D or rocker shaker)
- Molecular Imager (Bio-Rad ChemiDoc XRS+)
Software
- Image Lab 5.0 (Bio-Rad)
Procedure
文章信息
版权信息
© 2020 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Gnangnon, B., Peucelle, V. and Pierrot, C. (2020). Differential Fractionation of Erythrocytes Infected by Plasmodium berghei. Bio-protocol 10(11): e3647. DOI: 10.21769/BioProtoc.3647.
分类
微生物学 > 微生物生物化学 > 蛋白质
微生物学 > 微生物-宿主相互作用 > 体外实验模型
生物化学 > 蛋白质 > 分离和纯化
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
提问指南
+ 问题描述
写下详细的问题描述,包括所有有助于他人回答您问题的信息(例如实验过程、条件和相关图像等)。
Share
Bluesky
X
Copy link