- EN - English
- CN - 中文
Methodology for in vitro Assessment of Human T Cell Activation and Blockade
人体T细胞活化与阻断的体外评价方法
发布: 2020年06月05日第10卷第11期 DOI: 10.21769/BioProtoc.3644 浏览次数: 8175
评审: Lokesh KalekarLaura CampisiVikas Duhan

相关实验方案

研究免疫调控血管功能的新实验方法:小鼠主动脉与T淋巴细胞或巨噬细胞的共培养
Taylor C. Kress [...] Eric J. Belin de Chantemèle
2025年09月05日 3520 阅读
Abstract
Methods to test both the functionality and mechanism of action for human recombinant proteins and antibodies in vitro have been limited by multiple factors. To test the functionality of a recombinant protein or antibody, the receptor, the receptor-associated ligand, or both must be expressed by the cells present within the in vitro culture. While the use of transfected cell lines can circumvent this gap, the use of transfected cell lines does not allow for studying the native signaling pathway(s) modulated by the specific recombinant protein or antibody in primary cells. The present protocol utilizes sort purified CD14+ monocytes and T cells, both CD4+ T cells and CD8+ T cells, from healthy donors in a co-culture system. This methodology is particularly relevant for testing recombinant proteins or antibodies that are putative therapeutics for the treatment of autoimmune disease and cancer. While the current protocol focuses on co-cultures containing B7-H4 expressing monocytes plus either autologous CD4+ T cells or CD8+ T cells, the protocol can be modified for the user’s specific needs.
Keywords: PBMCs (外周血单核细胞)Background
The clinical use of checkpoint inhibitor therapy, e.g., anti-CTLA-4 and anti-PD-1, has proven useful as a cancer therapy to enhance the anti-tumor immune response (Curran et al., 2010; Gubin et al., 2014). While successful in patients that have failed frontline therapy, only approximately 20% of treated patients responded to checkpoint inhibitor antibody therapy. This inactivity of treatment in a patient may be multifactorial, and may also be reflective of the specific type of cancer that a patient presents with to the clinician (Podojil et al., 2020). B7-H4 expression by tumor cells and monocyte/macrophages present within the tumor is an additional putative mechanism by which tumor cells evade anti-tumor immune responses (Salceda et al., 2005; Simon et al., 2007; Qian et al., 2011; Abadi et al., 2013). Based on these published findings, the expression of B7-H4 within the tumor microenvironment, i.e., by the tumor cells and/or by infiltrating monocyte/macrophages, is hypothesized to allow for tumor cells to evade an anti-tumor immune response via both direct inhibition of effector T cell function and increasing regulatory CD4+ T cell function (Wei et al., 2011; Podojil et al., 2013).
The development of in vitro methods to determine the functionality of immunological therapeutics for the treatment of human disease has relied heavily on the use of cell lines. However, while there are similarities between the signaling pathways activated within human T cells lines (Jurkat and HuT78) and primary T cells isolated from PBMCs, there are distinct differences between the level of signaling pathway intermediate activation, the timing/longevity of signaling pathway intermediate activation, and the cytokine profile expressed between human T cells lines and primary T cells (Bartelt et al., 2009). For example, besides IL-2, both Jurkat and HuT78 cells are less depenent upon costimulation and secreted significantly lower levels of T cell effector cytokines, such as IL-4, IL-5, IL-17, TNF-α, and IFN-γ upon T cell receptor stimulation (Bartelt et al., 2009). This finding limits the utility of these cell lines when studying the ability of a recombinant protein or antibody to modulate cellular activities outside of T cell proliferation and IL-2 production.
The present method can be used to determine the functionality of therapeutics utilizing primary monocytes, CD4+ T cells, and CD8+ T cells purified from healthy human donors. Several cellular considerations for determining the functionality and identifying/validating the mechanism of action for a putative checkpoint inhibitor are required for assay development. The assay must be fit-for-purpose, robust, repeatable, and transferable between collaborating laboratories. In the present example for the study of anti-B7-H4 functional activity, cells that express B7-H4 must be present within the culture system, i.e., a macrophage population that has been pre-conditioned in the presence of recombinant human cytokines to induce the expression of B7-H4. Additionally, the B7-H4 receptor must be expressed by a target immune cell population, i.e., T cells. The B7-H4 receptor is expressed by a sub-set of both CD4+ T cells and CD8+ T cells following activation (Podojil et al., 2013). Therefore, an additional consideration for the present experimental design is that since the B7-H4 receptor is not present at high levels on freshly isolated T cells, the response rate within the co-culture system will display donor-to-donor variation.
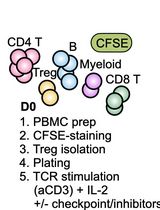
To this end, two general model are presented below. The first model utilized co-culture of B7-H4 expressing human macrophages with autologous purified CD4+ T cells, and the second model system utilized co-culture of B7-H4 expressing human macrophages with autologous purified CD8+ T cells. In brief, CD14+ monocytes are isolated from total PBMCs, and the CD14+ monocytes are cultured in the absence or presence of IL-10 and IL-6 for 3 days. Both CD4+ T cells and CD8+ T cells are isolated from the CD14- cell fraction of the healthy donor PBMC samples. The sort purified CD4+ T cells and CD8+ T cells are cryopreserved for future co-culture with the B7-H4 expressing macrophages. Figure 1 presents the number of each cell population isolated from twelve healthy donor PBMC samples. Following the initial 3 day macrophage culture in the presence of IL-10 and IL-6, the macrophages are harvested. The data show that macrophages cultured in the absence of IL-10 and IL-6 do not express B7-H4, while macrophages cultured in the presence of IL-10 and IL-6 do express B7-H4 (Podojil et al., 2020). On day 3, the cryopreserved autologous CD4+ T cells and CD8+ T cells are rapidly thawed, washed, and labeled with CFSE. The CD4+ T cells and CD8+ T cells are then co-cultured with IL-10 plus IL-6 conditioned macrophages in the presence of plate-bound anti-CD3 plus either a species and isotype matched Control antibody or anti-B7-H4. On day 6 of the assay (day 3 of macrophage-T cell co-culture) the level of CD4+ T cell and CD8+ T cell proliferation is assessed via flow cytometry (CFSE dilution), and the levels of secreted cytokine, such as IFN-γ, determined in the culture supernatants. While the use of transfected cell lines is a useful tool to quickly screen large numbers of recombinant proteins and antibodies for potential biological activity, the present protocol using primary cells allows for the validation of the initial in vitro cell line culture findings.
Materials and Reagents
- 50 ml conical tubes (CentriStar tube, orange cap) (Corning, catalog number: 430828 )
- 15 ml conical tubes (CentriStar tube, orange cap) (Corning, catalog number: 430790 )
- 6-well tissue culture plates (Costar, catalog number: 3516 )
- 96-well flat bottom tissue culture plates (Costar, catalog number: 3596 )
- Falcon Round-Bottom Tubes, 5 ml with snap cap (Corning, catalog number: 352054 )
- Human peripheral blood buffy coats (LifeSource)
- Ficoll-Paque PLUS (GE Healthcare, catalog number: 17-1440-03 )
- 1x DPBS without Ca2+ and Mg2+ (Gibco, catalog number: 2017-07)
- EDTA (0.5 M, pH 8.0) (Invitrogen, catalog number: 15575020 )
- Fetal calf serum (BenchmarkTM Fetal Bovine Serum) (Gemini Bio-Products, catalog number: 100-106 )
- Liquid nitrogen
- RPMI 1640 Medium without glutamine (Invitrogen, catalog number: 21870-100 )
- MEM non-essential amino acid solution (Sigma-Aldrich, catalog number: M7145-100 ml )
- L-glutamine-200 mM (100x) (Invitrogen, catalog number: 25030-081 )
- Penicillin-Streptomycin (Invitrogen, catalog number: 15140-122 )
- 2-mercaptoethanol (1,000x) (Gibco, catalog number: 21985-023 )
- HEPES (1 mM) (Gibco, catalog number: 15630-080 )
- Sodium pyruvate (100 mM solution with 8.5 g/L NaCl) (VWR, catalog number: 25-000-Cl )
- Trypan blue solution, 0.4% (Invitrogen, catalog number: 15250061 )
- CD14 Isolation Kit (Miltenyi, CD14 MicroBeads, human, catalog number: 130-050-201 )
- CD4 Isolation Kit (Miltenyi, CD4+ T Cell Isolation Kit, human, catalog number: 130-096-533 )
- CD8 Isolation Kit (Miltenyi, CD8+ T Cell Isolation Kit, human, catalog number: 130-096-495 )
- Human recombinant IL-6 (R&D Systems, catalog number: 206-IL/CF )
- Human recombinant IL-10 (R&D Systems, catalog number: 217-IL/CF )
- CellTraceTM CFSE Cell Proliferation Kit, for flow cytometry (Thermo Fisher, catalog number: C34554 )
- Anti-CD3 (clone OKT3) (Invitrogen, catalog number: 16-0037-81 )
- Viability stain (VID)–LIVE/DEADTM Fixable Aqua Dead Cell Stain Kit, for 405nm excitation (Thermo Fisher, catalog number: L34965 )
- Fc Block–Fc Receptor Binding Inhibitor Polyclonal Antibody, Functional Grade, eBioscienceTM (Thermo Fisher, catalog number: 16-9161-73 )
- Anti-CD4 APC, clone OKT4 (Thermo Fisher, catalog number: 17-0048-42 )
- Anti-CD8 APC, clone 3B5 (Thermo Fisher, catalog number: MHCD0805 )
- 1x DPBS + 2 mM EDTA (500 ml) (see Recipes)
- 1x DPBS + 5% FCS (500 ml) (see Recipes)
- Completed RPMI Medium (1,000 ml) (see Recipes)
- Cell Freezing Medium (2x stock) (see Recipes)
Equipment
- Pipettes
- Sterile hood
- -80 °C freezer with a Mr. Frosty Freezing container
- Centrifuge (Thermo ScientificTM SorvallTM ST 8FR Centrifuge)
- Incubator (Thermo ScientificTM FormaTM Steri-CultTM CO2 Incubators)
- Water bath
- Inverted phase contrast microscope
- autoMACS Pro Separator (Mitenyi Biotec)
- Flow cytometer (BD FACSCanto cytometer, BD Biosciences)
- MAGPIX Luminex 200 (Millipore)
Software
- FlowJo Version 10.5.3 (FlowJo)
- xPONENT software (Millipore)
Procedure
文章信息
版权信息
© 2020 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Podojil, J. R., Ifergan, I., Chiang, M., Meeks, J. J. and Miller, S. D. (2020). Methodology for in vitro Assessment of Human T Cell Activation and Blockade. Bio-protocol 10(11): e3644. DOI: 10.21769/BioProtoc.3644.
分类
免疫学 > 免疫细胞功能 > 淋巴细胞
免疫学 > 免疫细胞功能 > 巨噬细胞
细胞生物学 > 细胞分离和培养 > 共培养
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link