- EN - English
- CN - 中文
Determination of Ureides Content in Plant Tissues
植物组织中酰脲含量的测定
发布: 2020年06月05日第10卷第11期 DOI: 10.21769/BioProtoc.3642 浏览次数: 5257
评审: Amey RedkarSolmaz IraniMarisa Rosa
Abstract
The ureides allantoin and allantoate are the main organic nitrogen compounds transported in several legumes, predominantly from N2 fixation. Moreover, recent studies point out a remarkable role for allantoin during several stress responses of plants other than legumes. The goal of this protocol is to determine ureides concentration in different plant tissues. Ureides are extracted from plant material by boiling it in phosphate buffer. The allantoin and allantoate present in the supernatants are subjected to alkaline-acidic hydrolysis to glyoxylate. The glyoxylate is converted into glycoxylic acid phenylhydrazone, that is then oxidized to red-colored 1,5-diphenylformazan. The absorbance of supernatants is measured using a spectrophotometer at 520 nm. Ureides concentration can be inferred by using a glyoxylate calibration curve. Ureide quantification of different tissues of Arabidopsis thaliana and soybean plants were carried out following this protocol.
Keywords: Allantoin (尿囊素)Background
Determination of ureides is important for characterizing N2 fixation and assimilation in legume plants as well as stress and nutritional responses of non-ureidic plants like Arabidopsis (Brychkova et al., 2008; Watanabe et al., 2014; Irani and Todd, 2016 and 2018). The formation of allantoate is also useful for in vivo and in vitro determination of allantoinase activity (Duran and Todd, 2012). Techniques requiring expensive equipment, such as high-performance liquid chromatography (HPLC), are commonly used for ureide quantification while ethanol extraction is mostly employed for ureide recovery from plant tissues. The present protocol is based on flexible spectrometry that uses low volume of cheap reagents. The use of phosphate buffer instead of ethanol for ureide extraction and quantification greatly improved the reproducibility of the measurements, possibly by decreasing the interfering compounds that abound with the ethanol extraction.
Allantoin and allantoate present in plant extracts are converted to glyoxylate by alkaline-acidic hydrolysis (Vogels and Van der Drift, 1970). To carry out this protocol, three tubes (A, B and C) for alkaline and/or acid hydrolysis are prepared for each sample (Figure 1). The glyoxylate is converted into glycoxylic acid phenylhydrazone and is then oxidized by ferricyanide to form red-colored 1,5-diphenylformazan. The absorbance of supernatants is measured using a spectrophotometer at 520 nm. Allantoin content is obtained by subtracting the levels of glyoxylate resultant of allantoate degradation (tube B) from glyoxylate derived of allantoin alkaline-acid hydrolysis (tube C). Likewise, allantoate content can be inferred by the subtraction of basal glyoxylate levels (tube A) from glyoxylate converted from allantoate (tube B).
The described protocol was carried out for determine ureide concentration for different tissues, nutritional and stress conditions in Arabidopsis thaliana (Lescano et al., 2016 and 2020); and in roots and shoots of nodulating and non-nodulating soybean plants (Muñoz et al., 2016).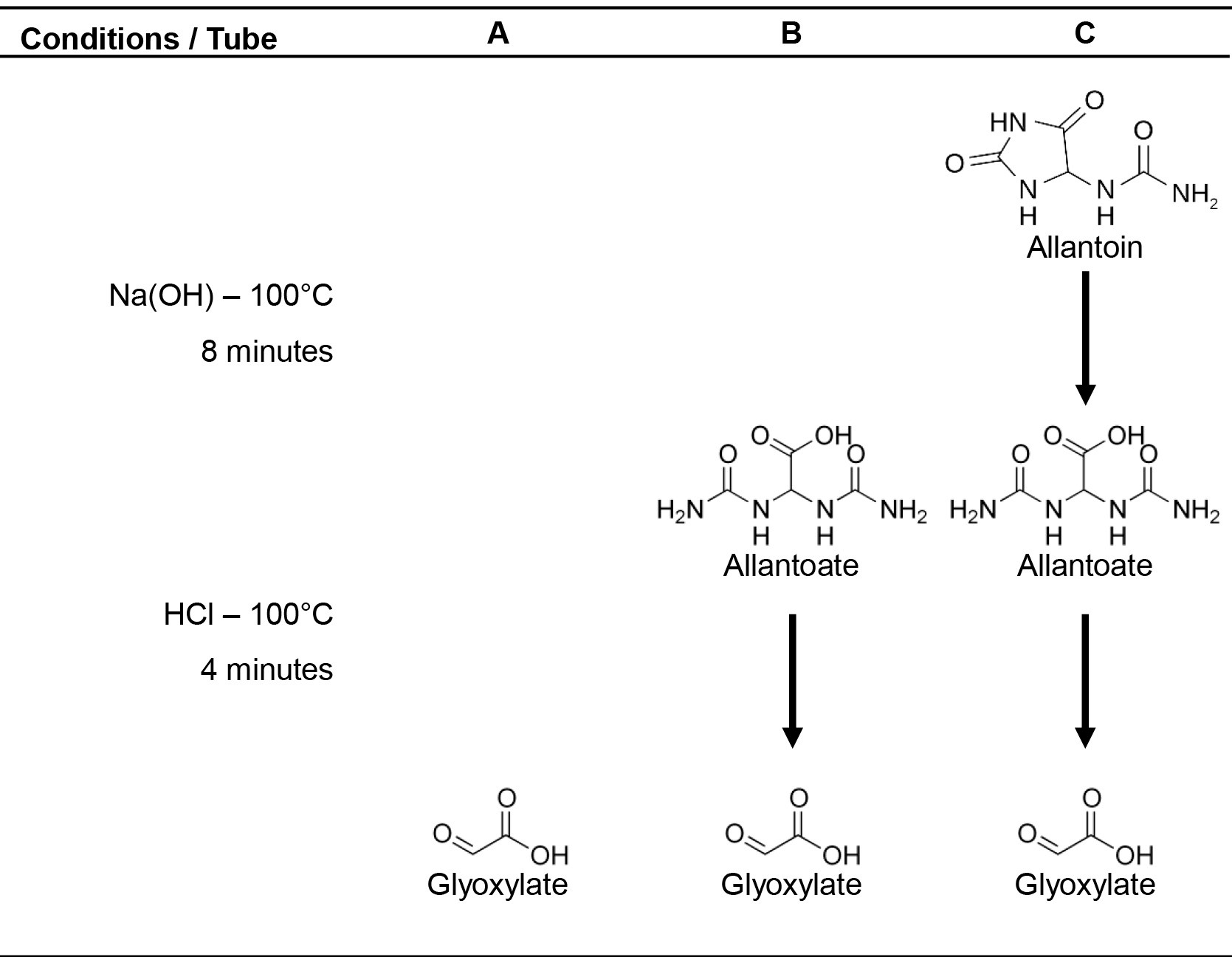
Figure 1. Alkaline-acidic hydrolysis of ureides
Materials and Reagents
- 1.5 ml microcentrifugue tubes (Eppendorf)
- 1.5 ml safe-lock microcentrifugue tubes (Eppendorf)
- 50 ml Falcon tubes
- Chemical resistant gloves
- Seeds (e.g., Arabidopsis)
- Growing medium [e.g., for Arabidopsis 0.5x MS (1% w/v agar) plates or soil: vermiculite (1:1) mix pots]
- Distillated water (dH2O)
- (Optional) Liquid air/N2
- Monopotassium phosphate (Cicarelli® Laboratories, catalog number: 1057211 )
- Dipotassium phosphate (Cicarelli® Laboratories, catalog number: 1015211 )
- Sodium hydroxide (NaOH) (Cicarelli® Laboratories, catalog number: 843211 )
- Hydrochloric acid (HCl) (Biopack, catalog number: 2000963208 )
- Phenylhydrazine (Merck, catalog number: 107251 ). Store at room temperature (RT) in the dark
- Potassium ferricyanide (Anedra, Research AG, catalog number: 7039 )
- Sodium glyoxylate monohydrate (Sigma-Aldrich, Merck, catalog number: G4502 )
- Extraction Buffer (see Recipes)
- Reaction Buffer (see Recipes)
Equipment
- Beaker
- (Optional) Mortar and pestle
- Plant growth chamber/greenhouse
- 60-65 °C incubator
- Analytical balance 0.5/0.0001 g (e.g., Mettler Toledo, model: AL204 )
- Centrifuge at 4°C with microliter rotor (e.g., Thermo Scientific, Sorval Biofuge Primo R)
- Ice bath
- Hot air bath at 100 °C
- Vortex mixer (e.g., Decalab)
- Fume hood
- VIS Spectrophotometer (e.g., Bioamerican Science, model: SP 2000 UV )
- Spectrophotometric plastic or glass VIS/UV-VIS semi-micro 0.75-1.5 ml cuvettes
Procedure
文章信息
版权信息
© 2020 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Lescano, I. (2020). Determination of Ureides Content in Plant Tissues. Bio-protocol 10(11): e3642. DOI: 10.21769/BioProtoc.3642.
- Lescano, I., Bogino, M. F., Martini, C., Tessi, T. M., González, C. A., Schumacher, K. and Desimone, M. (2019). Arabidopsis thaliana Ureide Permease 5 (AtUPS5) connects cell compartments involved in Ureide metabolism. Plant Physiol: pp.01136.2019.
分类
植物科学 > 植物生物化学 > 代谢物
植物科学 > 植物生理学 > 新陈代谢
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link