- EN - English
- CN - 中文
Enriched Environment Procedures for Rodents: Creating a Standardized Protocol for Diverse Enrichment to Improve Consistency across Research Studies
啮齿动物的环境丰容程序:创建提高研究一致性的多样化富集标准化协议
发布: 2020年06月05日第10卷第11期 DOI: 10.21769/BioProtoc.3637 浏览次数: 5030
评审: Alexandra GrosShaarika SarasijaAnonymous reviewer(s)

相关实验方案
基于 rAAV-α-Syn 与 α-Syn 预成纤维共同构建的帕金森病一体化小鼠模型
Santhosh Kumar Subramanya [...] Poonam Thakur
2025年12月05日 1589 阅读
Abstract
Exposure to environmental enrichment has beneficial effects on learning and memory, diverse neurobiological effects, and promotes recovery of function after brain injury. The effect of enrichment is produced by a combination of increased social interaction, physical activity, spatial complexity, and novelty. Procedures in the literature have, however, been idiosyncratic with poor consistency in the manner or extent to which protocols provide consistent enrichment. We provide an environmental enrichment protocol that can be easily replicated with minor details determined locally so that animals across cohorts and cages all experience a comparable level of enrichment. Procedures are outlined to generate and use a daily pool of suitably varied objects using a standardized format, with objects systematically varied up to a 40-day continuous period. Together with using a large group of rats in a suitably-sized cage, and regular shifting of the position of food and water and cage location, these procedures have produced robust effects in different laboratories and rat strain, thereby improving comparisons within and across laboratories. Non-enriched comparisons can vary, but typically would include grouped animals in standard laboratory housing without objects and with stable food and water locations. Enrichment is a safe non-pharamacological tool to examine behavioral and neurobiological processes in animal models of the lifespan, brain dysfunction and injury.
Keywords: Enrichment (富集)Background
Enriched environments are a proven therapeutic tool to ameliorate cognitive and affective deficits associated with age-related decline and after many types of brain injury (van Praag et al., 2000; Will et al., 2004; Nithianantharajah and Hannan, 2006; Pang and Hannan, 2013; Alwis and Rajan, 2014; Hannan, 2014). This includes improved cognitive reserve, recovery from relatively diffuse brain injury, such as stroke, traumatic brain injury and various neurodegenerative diseases (Johansson, 2004; Pang and Hannan, 2013; Alwis and Rajan, 2014; Hannan, 2014), as well as from acute localized lesions in cortex, hippocampal formation and thalamus (Will and Kelche, 1992; Will et al., 2004; Harland et al., 2014; Dalrymple-Alford et al., 2015). These effects are likely to be mediated by increased activation of multiple brain networks, including their recruitment during behavioral tasks. Neurobiological effects range from the size and morphology of brain regions to the survival and complexity of neurons, adult neurogenesis, enhanced cell excitability, synaptic plasticity, place cell function, and a wide array of neuroprotective molecular responses that reflect multiple genetic processes, including gene-environment interactions, neuroinflammation and trophic factors (Rampon et al., 2000a and 2000b; Will et al., 2004; Leger et al., 2012; Bonaccorsi et al.,2013; Briones et al., 2013; Eckert and Abraham, 2013; Alwis and Rajan, 2014; Hirase and Shinohara, 2014; Bilkey et al., 2017; Kempermann, 2019; Ohline and Abraham, 2019). These benefits of enrichment are thought to arise from a combination of increased exercise, socialization, and novelty, producing stronger effects than each of these components administered individually (Einon et al., 1980; Will et al., 1986; Olson et al., 2006; Cracchiolo et al., 2007; Sozda et al., 2010). Environmental enrichment is often used as general ‘cognitive treatment’ and produces behavioral and cognitive enhancements in young and old intact or ‘sham-operated’ rats (Gonzalez-Ramirez et al., 2014; Harland et al., 2014). Laboratories that study spatial navigation, memory, or neurogenesis, often raise experimental animals in enriched environments as a cognitive enhancer, for example, groups that study in vivo neuronal electrophysiology (e.g., Moser group, Kavli Institute, Norway; Jeffery group, University College London, United Kingdom).
An enriched environment for rats or mice in a laboratory setting generally includes a moderate (10-12) group of animals housed in a large cage in which they are exposed to stimulation far beyond that afforded by the standard, generally barren laboratory cage that often houses 3 to 6 animals (Simpson and Kelly, 2011). Explicit environmental stimulation is provided by adding multiple objects within the enrichment cage. However, these objects are sometimes infrequently or randomly varied in many studies. More consistent enrichment should regularly introduce new objects and new configurations, preferably on a daily basis. This more systematic approach provides informal, but varied and multisensory cognitive stimulation and learning, together with physical and social experiences. Unlike natural (feral) conditions, laboratory enrichment establishes a safe complex environment that has relatively stable social conditions but in which the inanimate physical conditions are frequently changing. Environmental enrichment procedures, such as the number of rats per cage, cage equipment and space, and especially the type and frequency of objects, have often varied markedly across enrichment studies (Simpson and Kelly, 2011). A range of different exposure periods has been implemented, with the majority of studies focusing on enrichment for 30 or 40 days. Periods as short as 4 days or non-continuous exposures of just several hours a day have also been used (Kolb and Gibb, 1991; Wallace et al., 1992; Bindu et al., 2005). The current protocol outlines a systematic method to produce standardized continuous enrichment paradigms for use with rats (or mice) to allow different cohorts of animals to experience comparable enrichment that can be easily replicated in most laboratories to achieve more consistent enrichment procedures. A rigidly standardized protocol across all labs is not feasible, but our protocol provides a clear guide of how a greater degree of standardized enrichment can be achieved than is currently the case. It describes a systematic approach in the frequency, variation and placement of objects to standardize this aspect of the enrichment procedure across experiments within any given lab and to encourage greater similarity across laboratories with respect to object-based enrichment protocols.
Materials and Reagents
- Bedding
Bedding should be changed at least once per week; for us this was once per week just after the cages were washed. We used Natures Flame pellet fuel (Nature’s Flame Limited, New Zealand 1PSI) for bedding in standard (control) cages, which breaks down into sawdust over a few days. The same bedding, but already in sawdust form, was used in the enrichment cages, to help facilitate the positioning of objects; this was created by soaking and breaking up the Natures Flame pellets in a little water and then leaving them to dry. Presoaking of the bedding material for the standard-housed rats could also be used, but the additional technician time required for the standard cages is not necessary. Any suitable laboratory-standard animal bedding is suitable from your supplier and this does not need to be varied between enriched and standard caging. - Rats
We have used male hooded PVGc rats (e.g., Harland et al., 2014), as well as female hooded PVGc rats, with this protocol, which were bred in-house in our Facility. Only single-sex housing is used in the enrichment literature. If female rats are used, we have not found it necessary to control for the estrus cycle; this decision may depend on the particular study, especially where hormonal changes are relevant. Our protocols have been shown to be effective in Long-Evans rats (Ali et al., 2017). Any persistently aggressive animal should be removed, and more frequently monitored if a given strain is known in this regard. Our rat strain was originally supplied by an Animal Resources Centre in Australia. A wide variety of different rat (and mouse) strains have been used in the literature; direct comparisons of rat strain for enrichment effects are uncommon. Although it is common in the current literature to provide the weight of rat, for enrichment the more relevant variable is the actual age of the animals used (an estimate of age on arrival can be provided by your supplier if in-house breeding is not employed). The age of rat used would be determined by the specific experimental questions under study. In standard laboratory housing conditions, some strain of rat (e.g., Long-Evans; some albino strains) can show evidence of obesity after young adulthood (Tordoff et al., 2008). Obesity in these strains needs to be countered by advance planning of mild food restriction when young adults and this may require comparisons of weight gain across the two housing conditions. Enriched rats are generally lighter than standard-housed rats, but marked differences in weight should be avoided in our view by mild food restriction in both groups. - Enrichment objects
A large collection of objects with at least four copies of each–see Step A2 for guide on collecting suitable objects. Only clean objects should be introduced. - Clips
An assortment of clips to connect enrichment objects to each other or to the walls of the cage. For example, we used: Medium fold-back paper clips (e.g., Medium Binder Clip, Staples, catalog number: 103549 ) and S Hooks (e.g., 1” S Hook, Staples, catalog number: 272145 ). - Detergent
To clean enrichment objects daily. We used Pyroneg powder (2-3 grams per litre; Thermo Fisher, catalog number: DIVHH13231 ). - Wooden blocks
Non-toxic small hard-wood blocks from your local supplier (8.5 x 9.4 cm, and 1.5 cm thick or similar), are used for rats to gnaw on after initially sterilized. These blocks minimize any damage to objects. Any contaminant-free wooden blocks would be suitable (e.g., Wood Gnawing Blocks, Bio-Serv, catalog number: K3512 ).
Equipment
- Large enrichment cages
We used locally built wire-mesh hot-dip galvanized cages to house 10-12 rats (maximum of 12 in our case). The cage was 85 cm long by 60 cm wide by 30 cm high, with a solid metal floor and two hinged mesh lids. Figures 1A-1B show our enrichment cage. Figures 1C-1D shows two groups of rats participating in the enriched environment (the environment changes daily). Enrichment cages can be constructed by local manufacturers (as in our case) or custom built by cage suppliers. Another option could be to use suitable commercially available cages for pet rats, hamsters or birds. Multi-level cages may be used, but these are regarded as a modification to standardized enrichment protocols. The size of cage and number of rats should accommodate the strain being used and local conditions and requirements. The floor area per animal for the enrichment cage (height x width of floor divided by number of animals; 425 cm2 in our protocol with 12 male rats) is a little greater than the floor area per animal in the standard laboratory cages (375 cm2 in our protocol with 4 male rats). The minimum floor space per rat based on weight should follow the National Research Council: Guide for the use and Care of Laboratory Animals (2011) (Reference 23) guidelines: e.g., for rats < 100 g floor area per animal > 109.6 cm2, for rats < 200 g floor area per animal > 148.35 cm2, for rats < 300 g floor area per animal > 187.05 cm2, for rats < 400 g floor area per animal > 258 cm2, for rats < 500 g floor area per animal > 387 cm2, for rats > 500 g floor area per animal > 451.5 cm2. The guide also recommends that cages should be < 17.8 cm high, but we recommend < 27 cm high if possible, to allow rats to stand fully upright (Makowska and Weary, 2016). Very large rats (> 900 g) may need even more space, such as 1000 cm2 floor area per animal and 30 cm cage height (see Lawlor, 1990). “Crowding” in our enrichment cages does not occur because rats have the whole area to explore and rats generally socially interact and aggregate together no matter what the size of cage provided. For example, object #13 (Appendix 1 in Supplemental) shows a 15 x 15 x 15 cm clear Perspex box with holes for entry/exit; there may be 2 to 6 rats who self-select to spend periods of time within this box. Within reason, social interaction is more critical than the space afforded per rat within the cage. However, rats in enrichment should be monitored for any overt signs of stress (behavioral changes, vocalization, chromodacryorrhea, unexpected weight loss). Plasma corticosterone response could also be monitored as an analog of stress; other researchers using our enrichment protocol reported relatively low levels of intracardial plasma corticosterone (150 ng/ml) and no differences on this measure between enriched and standard housed rats at the end of a 40-day continuous period of differential housing (see Supplementary Figure 2 from Ali et al., 2017). Small variations in cage size is unlikely to have a substantial effect on the results. Our cages were specifically designed to be able to be housed within racks in our colony rooms, so it is important to consider these kinds of restrictions when ordering cages. Cages need to be high-grade stainless steel and be able to withstand repeated exposure to an industrial (walk-through) cage washer. Alternatively, iron or steel cages can be hot-dip galvanized in zinc to make them rustproof.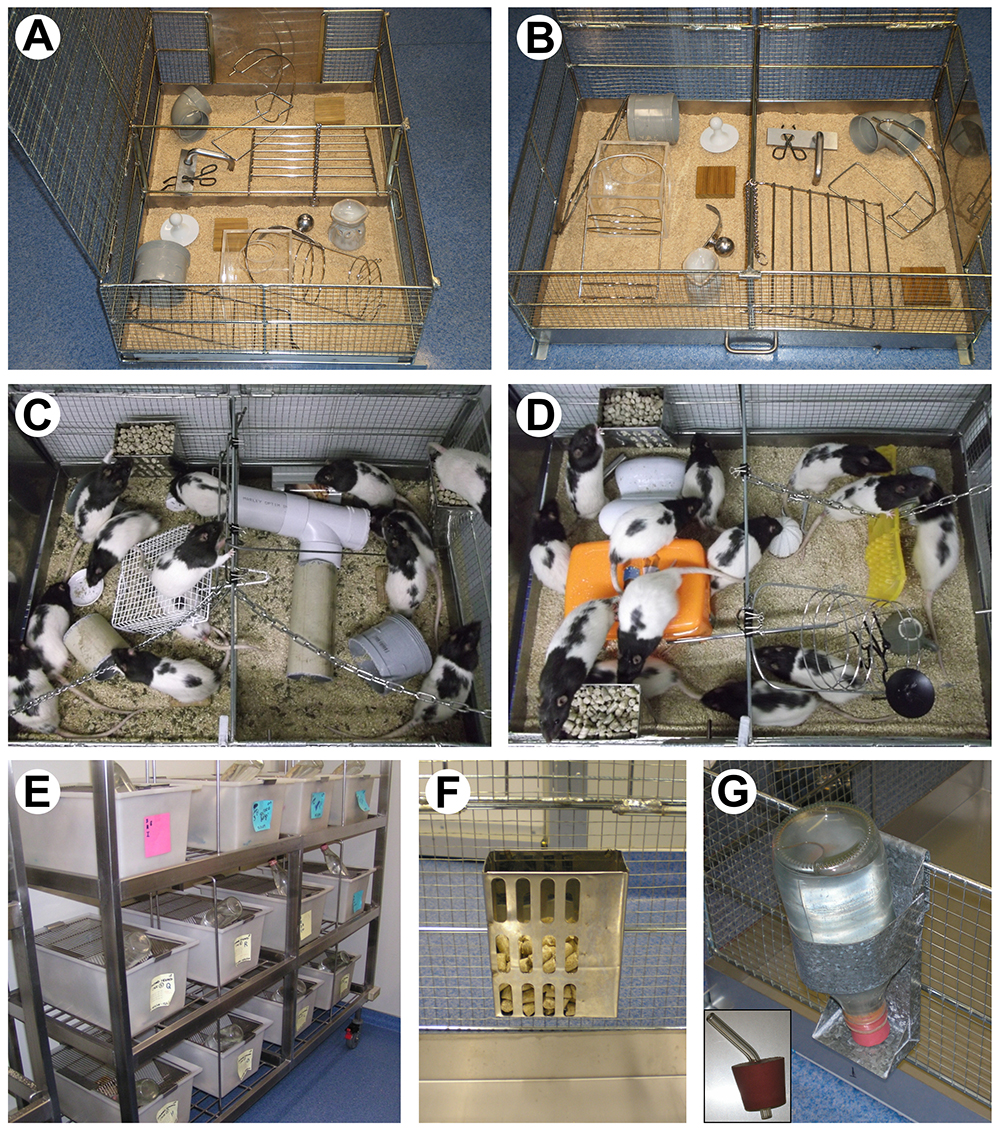
Figure 1. Examples of the enrichment setup used in our laboratory. A and B. The enrichment cage, containing an example of the daily configuration of objects, is shown from (A) the side, and (B) the front. C and D. Rats participating in environmental enrichment in two different object configurations are shown in (E). E. Example of standard cages in the colony room. F. Moveable metal food hoppers that could be attached at different heights and positions within the enrichment cages. G. Glass water bottle mounted in moveable metal holder; the bottle cap (shown in insert) was hard rubber and contained a glass spout angled into the cage. - Standard cages
We used opaque (autoclavable) plastic cages to house 3-4 rats. These standard housing cages were 50 cm long by 30 cm wide by 22 cm high, with stainless steel grille lids. Figure 1E shows our standard cages on a stainless-steel rack in a colony room. Any kind of regular laboratory cage, which houses 2-6 animals (we usually have 3 or 4), would be suitable for use as the “standard” housing condition. If there is a local preference that one or two objects be placed in this cage and remain unchanged (other than for cleaning) we do not regard this as “enrichment”. - Food hoppers
Figure 1F shows the food hoppers allowing easy relocation of food around the inner walls of the enrichment cage (e.g., Ancare mouse or rat feeders, catalog number: MF342/RF452 ). Any standard laboratory rat chow is suitable to use as food for both the enriched and standard housing. - Metal bottle holders
Figure 1G shows glass bottles (600 ml) and metal bottle holders used in our studies allowing easy relocation of water position across days (e.g., Ancare sure lock bottle holders, catalog number: H-100 to H-102). Any bottle compatible with the holder would be suitable. The drinking spout should be rodent compatible (i.e., with one or more metal ball bearings to hold water contents) and long enough to protrude inside the cage wall. - (Optional) Light
We use a reversed light cycle in the colony room so that behavioral testing is conducted in their dark period, that is, when rats are naturally more often active. The test environment can of course have lighting, because the modern-day natural environment would also include lit areas during the true night-time period. This is a local preference issue.
Note: All equipment was cleaned once a week using an industrial rack-sized cage wash, but any suitable cleaning arrangement can be used.
Procedure
文章信息
版权信息
© 2020 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Harland, B. C. and Dalrymple-Alford, J. C. (2020). Enriched Environment Procedures for Rodents: Creating a Standardized Protocol for Diverse Enrichment to Improve Consistency across Research Studies. Bio-protocol 10(11): e3637. DOI: 10.21769/BioProtoc.3637.
分类
神经科学 > 行为神经科学 > 认知
神经科学 > 神经系统疾病 > 动物模型
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link