- EN - English
- CN - 中文
Extracellular Vesicles Tracking and Quantification Using CT and Optical Imaging in Rats
大鼠细胞外囊泡的CT和光学成像追踪与定量研究
发布: 2020年06月05日第10卷第11期 DOI: 10.21769/BioProtoc.3635 浏览次数: 6016
评审: Karem A CourtAlexandros C KokotosLuis Alberto Sánchez Vargas
Abstract
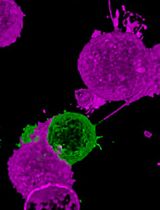
Exosomes, a subtype of extracellular vesicles, are nanovesicles of endocytic origin. Exosomes contain a plethora of proteins, lipids, and genetic materials of parent cells to facilitate intercellular communications. Tracking exosomes in vivo is fundamentally important to understand their biodistribution pattern and the mechanism of biological actions in experimental models. Until now, a number of tracking protocols have been developed, including fluorescence labeling, bioluminescence imaging, magnetic resonance imaging, and computed tomography (CT) tracking of exosomes. Recently, we have shown the tracking and quantification of exosomes in a spinal cord injury model, by using two tracking approaches. More specifically, following intranasal administration of gold nanoparticle-encapsulated exosomes to rats bearing complete spinal cord injury, exosomes in the whole central nervous system were tracked by using microCT, and quantified by using inductively coupled plasma and flame atomic absorption spectroscopy. In addition, optical imaging of fluorescently labeled exosomes was performed to understand the abundance of migrating exosomes in the spinal cord lesion, as compared to the healthy controls, and to further examine their affinity to different cell types in the lesion. Thus, the protocol presented here aids in the study of exosome biodistribution at both cellular and organ levels, in the context of spinal cord injury. This protocol will also enable researchers to better elucidate the fate of administered exosomes in other models of interest.
Keywords: Exosomes (外泌体)Background
Exosomes are natural nanovesicles (30-150 nm in diameter) of endosomal origin, secreted by a variety of cells including mesenchymal stem cells (MSCs) (Yang et al., 2018). Exosomes carry proteins, lipids and genetic materials of parent cells, and are capable of inducing a multitude of biological effects to adjacent or distal cells and tissues (Valadi et al., 2007; van Niel et al., 2018). Due to their innate advantages, such as low immunogenicity, potential of crossing blood brain barrier, and amenability to the loading of exogenous cargos, exosomes have emerged as an alternative to cell-based therapeutics in the treatment of cancer, neurodegenerative and many other diseases (Alvarez-Erviti et al., 2011; Kamerkar et al., 2017; Guo et al., 2019; Yong et al., 2019). While exosomes have gained increasing interest as diagnostic and therapeutic vehicles, the knowledge of their in vivo behavior is limited, which restricts the clinical translation of exosome therapy. Thus, advanced imaging approaches for in vivo tracking of exosomes are highly anticipated.
To elucidate the in vivo behavior of exosomes, several strategies have been developed. Among them, optical imaging represents the most commonly used exosome tracking method. Up until now, organic dyes, immunofluorescent reporters, genetically encoded fluorescent proteins, or fluorescent nanomaterials have been used to label exosomes (Pegtel et al., 2010; Alvarez-Erviti et al., 2011; Suetsugu et al., 2013; Lai et al., 2015; Jiang et al., 2018; Shen et al., 2018; Betzer et al., 2019). Optical imaging allows hgh-throughput efficiency, facilitating the observation of the interplay between exosomes and recipient cells. However, this technique has several drawbacks, such as artifacts due to lipophilic dye aggregation (Grange et al., 2014) or presence of autofluorescence background (Faruqu et al., 2019), as well as limited tissue penetration depth (Shen et al., 2018). Magnetic resonance imaging (MRI) constitutes another promising tracking methodology, featured by high spatial resolution with anatomical details and radiation-free operation (Busato et al., 2017). Exosomes can be loaded with superparamagnetic iron oxide nanoparticles (SPIONs), and tracked in animal models using MRI (Hu et al., 2015; Busato et al., 2016; Jia et al., 2018). MRI, however, has inherent disadvantages, including susceptibility to movement artifacts, long image acquisition, and high cost. Computed tomography (CT) is a powerful, and widely used imaging modality which offers high spatial and temporal resolution. Recently, Betzer et al established a protocol for labeling exosomes with gold nanoparticles (GNPs) for tracking exosome migration in vivo using CT (Betzer et al., 2018). This technology has been successfully utilized to track the biodistribution of intranasally delivered exosomes derived from mesenchymal stem cell (MSC), in rodents with focal brain ischemia (Betzer et al., 2017), various brain pathologies (namely, stroke, Parkinson’s disease, Alzheimer’s disease, or autism) (Perets et al., 2019), or spinal cord injury (SCI) (Guo et al., 2019). Guo et al combined optical imaging and CT neuroimaging modalities, to examine the biodistribution of intranasally administered MSC-derived exosomes in rats with complete SCI (Guo et al., 2019). Coupled with quantitative methodologies, i.e., inductively coupled plasma (ICP), flame atomic absorption spectroscopy (FAAS), and analysis of immunofluorescently stained tissues, a more comprehensive exosome tracking was demonstrated, unraveling the interactions between exosomes and different cell populations in the spinal cord, and the localization of exosomes in several major organs, in the context of SCI.
Multimodal imaging modalities are more anticipated than single imaging model with regard to elucidating the behavior of exosomes in vivo through complementary advantages. The protocol presented here can be expanded to investigate how exosomes behave in other diseased models.
Materials and Reagents
- 0.22 μm filter
- 70 ml polycarbonate tubes
- Permanent marker
- Coverslips
- Single-neck round-bottom flask (Sigma-Aldrich, catalog number: Z414514 )
- 0.45 μm syringe filters (CSI Analytical Innovations, catalog number: 30103 )
- Spinal cord hook (Fine Science Tools, catalog number: 1016212 )
- #10 blade (Albion, catalog number: 15478491 )
- 5-0 Absorbable sutures (Assut, catalog number: 704031 )
- 4-0 sutures (Assut, catalog number: 6543 )
- Female Sprague Dawley rats, 200-250 gram, 8-10 weeks old
- Bone marrow mesenchymal stem cells (Lonza, catalog number: 10HU-217 )
- DMEM medium (Biological Industries, catalog number: 01-056-1A )
- L-glutamine (Biological Industries, catalog number: 03-022-1B )
- Penicillin-streptomycin-neomycin (Biological Industries, catalog number: 03-034-1B )
- MEM-eagle non-essential amino acid (Biological Industries, catalog number: 01-340-1B )
- Heparin (Sigma, catalog number: H9267-1MG )
- Platelets lysate (Rabin Medical Center, Israel)
- PKH26 dye (Sigma, catalog number: PKH26GL-1KT )
- Deuterium-depleted water (DDW) (Thermo Scientific)
- Sodium Hydroxide (NaOH) (Sigma, catalog number: 1310-73-2 )
- Oleic acid (Sigma, catalog number: 112-80-1 )
- Palmitic acid (Chem-Implex Int'l, catalog number: 14036 )
- HAuCl4 (Sigma, catalog number: 27988-77-8 )
- Ascorbic acid (Sigma, catalog number: 50-81-7 )
- O-(2-Carboxyethyl)-O′-(2-mercaptoethyl)heptaethylene glycol (PEG7) solution (Sigma, catalog number: 866889-02 )
- n-hexane (Bio-Lab, catalog number: 110-54-3 )
- HCl solution (Deajung, catalog number: 764701-0 )
- N-Hydroxysulfosuccinimide sodium salt (NHS) (Sigma, catalog number: 106627-54-7 )
- N-(3-Dimethylaminopropyl)-N-ethylcarbodiimide hydrochloride (EDC) (Sigma-Aldrich, catalog number: 25952-53-8 )
- Glucose-2 (2GF) (D-(+)-glucosamine hydrochloride (Sigma-Aldrich, catalog number: 66-84-2 )
- Acetone (Romical, catalog number: 19-009001-72 )
- Ketamine (Vetoquinol, catalog number: 8c0903c )
- Xylazine (Eurovet, catalog number: 0 84697 )
- Isoflurane (Abbott Laboratories, catalog number: 6041795 )
- Eye ointment (Alcon, catalog number: 16D12E )
- Buprenorphine (Medi-market, catalog number: V82806J )
- 0.9% saline (Baxter, catalog number: 18K29E0B )
- Polydine-iodine (Dr. Fisher, catalog number: B.N.D0410PT20.2 )
- Phosphate buffered saline (PBS) (Gibco, catalog number: 14200067 )
- 4% Paraformaldehyde (ChemCruz, catalog number: sc281692 )
- Sucrose (Baker Analyzed, catalog number: 57501 )
- Triton X-100 (Biolab, catalog number: 20180501 )
- Tween-20 solution (Sigma, catalog number: P2287 )
- Primary antibodies
- Secondary antibodies
Goat anti rabbit 647 (LifeTechnologies, catalog number: A21244 ) - DAPI (Sigma, catalog number: D9592 )
- Bovine serum albumin (BSA) (Millipore, catalog number: 820451 )
- Sucrose solution (see Recipes)
- Blocking solution (see Recipes)
- Tween solution (see Recipes)
Equipment
- Tunstem Carbide Noyes microscissor (Fine Science Tools, catalog number: 1551412 )
- Friedman-Pearson Rongeur (Fine Science Tools, catalog number: 1602114 )
- Heating pad (Gaymar Industries, model: TP702 )
- Electric shaver (Oster Golden A5 Two-speed, model: 0 7800550002 )
- Inhalation set-up (MatrxTM VIP 3000 Calibrated Vaporizer, catalog number: V1419556 )
- Funnel (Separatory funnel 250 ML/glass stopcock) (Sigma, catalog number: 64803-U )
- Vacuum pump (Millivac-Mini Vacuum Pump) (Merck, catalog number: XF5423050 )
- Inductively-Coupled-Plasma Optical-Emission-Spectrometry (ICP-OES) (Santa-Clara, USA, Agilent, model: 5100VDV )
- Flame atomic absorption spectroscopy (Agilent Technologies, model: SpectrAA 140 )
- Micro-CT scanner (Skyscan High Resolution, model: 1176 )
- Cryostat (Germany, Leica, model: CM1850 )
- Confocal microscope (ZEISS, model: LSM700 )
- Ultracentrifuge (Beckman, model: Optima XE-90 Ultracentrifuge )
Software
- Fiji: ImageJ
- GraphPad Prism 7
- Matlab
- SkyScan CT-Voxel (“CTVox”)
- SkyScan CT-Volume (“CTVol”)
Procedure
文章信息
版权信息
© 2020 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Guo, S., Betzer, O., Perets, N., Landau, S., Offen, D., Popovtzer, R. and Levenberg, S. (2020). Extracellular Vesicles Tracking and Quantification Using CT and Optical Imaging in Rats. Bio-protocol 10(11): e3635. DOI: 10.21769/BioProtoc.3635.
分类
细胞生物学 > 细胞器分离 > 外来体
细胞生物学 > 细胞成像 > 活细胞成像
分子生物学 > 纳米颗粒 > 植物源纳米颗粒
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link