- EN - English
- CN - 中文
RNA Extraction from Ears and Draining Lymph Nodes of Mice Infected with Leishmania amazonensis
亚马逊利什曼原虫感染小鼠耳及引流淋巴结RNA的提取
发布: 2020年06月05日第10卷第11期 DOI: 10.21769/BioProtoc.3633 浏览次数: 5515
评审: Alexandros AlexandratosMarieta RusevaAnonymous reviewer(s)

相关实验方案

利用气相色谱法定量分析小鼠血清、结肠管腔内容物和粪便中短链脂肪酸
Willian Rodrigues Ribeiro [...] Caroline Marcantonio Ferreira
2018年11月20日 14596 阅读
Abstract
Parasites of the genus Leishmania infect the mammalian hosts, including mice and humans and cause cutaneous or visceral leishmaniasis depending upon the parasite species transmitted by the vector sandfly. Leishmania amazonensis is one of the Leishmania species responsible for the cutaneous form of the disease. We have inoculated with these parasites the ear dermis of mice. RNA preparations were performed from fragmented tissues using a buffer containing guanidin isothiocynate (RLT buffer, RNeasy Mini Kit, Qiagen, SAS, France) and β-mercaptoethanol. Both reagents facilitate the isolation of intact RNA from tissues and the use of the RNeasy Kits present with several advantages that facilitate the isolation of pure non-degraded total RNA: i) This method allows to avoid the presence of phenol in the RNA extraction buffer, commonly used in alternative protocols; ii) Moreover Diethylpyrocarbonate (DEPC) treatment of glassware, to avoid RNAses contamination of the samples, is not required with this protocol; iii) Finally, it is a fast procedure and the isolated total RNA may be concentrated in a small volume thus facilitating its use for downstream experimental procedures.
Keywords: RNA isolation (RNA分离)Background
Pathogens of the genus Leishmania cause vector born zoonotic diseases with a variety of clinical manifestations and a variability in disease severity (Mansueto et al., 2007). The cutaneous form of leishmaniasis is caused by several Leishmania species including Leishmania amazonensis (McMahon-Pratt and Alexander, 2004; Christensen et al., 2019).
The existence of mouse models, with high, intermediate or no susceptibility to the cutaneous form of leishmaniasis have contributed to the development of experimental protocols for parasite dissemination (de Oliveira Cardoso et al., 2010). The parasites are naturally transmitted to the mammalian hosts by the bite of infected phlebotomine sandflies of the genus Lutzomyia (Bates, 2007). Thus transmission protocols use the ear dermis, the dorsal skin or the hind footpad (Schuster et al., 2014). In our protocol the Leishmania amazonensis parasites were inoculated through the ear dermis of the mouse, a method that closely imitates the natural transmission of the parasites by the sandfly (Giraud et al., 2019b). In addition, the ear pinna is appropriate for phenotypic evaluation of the lesion as well as for the isolation of nucleic acids from the inoculation sites, for two reasons: 1) parasites, in their infectious path, induce two distinct phases: the first, is a clinically silent phase characterized by the absence of lesions and the increase of parasite load and the second phase, during which visible lesions develop, and are associated with immune cell infiltration at the site of inoculation (Liu and Uzonna 2012; Giraud et al., 2019a). 2) The immune cells expand in the draining lymph nodes, which are also used for nucleic acids preparation (Cortes et al., 2010). Therefore the immune response of the host to the parasites can be studied on the site of inoculation and on the secondary immune adjacent tissue, the ear draining lymph nodes.
Leishmania amazonensis infection triggers an inflammatory response characterized by the presence of macrophages on the lesion sites. The parasites develop in the resident dermal macrophages (MF) and in Dendritic cells (DC) (Liu and Uzonna, 2012). The mechanisms of the parasite adaptation in the mammalian phagocytes and tissues remain elusive. Macrophages do not prevent the proliferation of the infection but on the contrary are the resident cells for these parasites.
We have studied the immune response of the host to Leishmania amazonensis infection in mice (Giraud et al., 2019b). Parasites transmitted through the ear dermis induce the immune response of the adjacent lymph nodes. RNAs prepared from the ear lesions and the lymph nodes were used to address the immune-related gene expression prior and after infection with the parasites. Several methods exist for preparation of ribonucleic acids. The classical protocols use guanidium isothiocyanate, a chaotropic agent that disintegrates the cellular structures and dissociates rapidly the nucleoproteins from the nucleic acids (Reid, 1991). RNases are also inactive in the presence of 4 M guanidium thiocyanate. Beta-mercaptoethanol (β-ME) is added to reduce RNA degradation by RNases. These protocols require disposable sterile glassware and plastic ware for the preparation of non-degraded RNA. Often pretreatment with diethylpyrocarbonate (DEPC) (0.1%) is used to treat glassware for 12 h at 37 °C and then heating at 100 °C for 15 min to remove any traces of diethylpyrocarbonate. The cellular proteins are removed from the nucleic acids preparations by extractions with phenol/chloroform. Ethanol precipitation of the isolated RNAs is then required and the nucleic acids are obtained by precipitation in the presence of 0.3 M sodium acetate. Our method benefits from the existence of a simplified protocol provided by the Qiagen kits. The methodological advantages of this protocol are as follows: 1) the method is simplified and quick, 2) there is no need to use DEPC, 3) phenol extraction of the nucleic acids from the proteins is not required, 4) the silica columns for separation of the nucleic acids from the cells or tissues constituents are very efficient, 5) RNAs are at a correct concentration and ethanol precipitation is not necessary and finally this method yields a high quality of RNAs. Whilst accompanied protocols are provided by the Qiagen kits, the additional details in the description of our method, applied to the mouse ear and lymph nodes, completes the methodology of the corresponding published article (Giraud et al., 2019b), presents with an interest for the isolation of pure non-degraded RNAs and may be useful to other investigators.
Materials and Reagents
- 60 x 15 mm, Cell culture-treated Permanox Petri Dish sterile (Thermo Fisher Scientific, NuncTM, catalog number: 150340 )
- Pipette Barrier (Filter) tips (Certified Free for RNases, DNases, sterile) (Thermo Fisher Scientific, Invitrogen, catalog numbers: AM126-45 (10 μl), AM126-35 (20 μl), AM126-50 (200 μl), AM126-60 (1,000 μl)
- 2 ml and 1.5 ml tubes (Eppendorf, Fisher Scientific, catalog number: 3810X )
- 2 ml hard tissue homogenizing tubes, containing 2.8 mm ceramic (zirconium oxide) beads CK28 (Bertin Instruments, catalog number: P000911-LYSK0-A , for Precellys tissue homogenizer, see Equipment 1)
- Glass Pasteur Pipettes (Merck, catalog number: CLS7095D5X )
- Adult C57BL/6J and B6(Cg)-Spp1tm1Blh/J mice (8-12 weeks of age)
- Phosphate buffered saline (PBS BioPerformance Certified, pH 7.4, Sigma-Aldrich, catalog number: P5368-10PAK , storage at room temperature)
- β-Mercaptoethanol (β-ME), Molecular Biology Grade (Millipore, Merck, Calbiochem, catalog number: 444203-250ML or alternatively dithiothreitol (DTT) may replace β-ME [Mommaerts et al., 2015])
- RLT (RNA lysis buffer, containing guanidine thiocyanate) (Qiagen, catalog number: 79216 )
- RW1 (RNA wash buffer containing ethanol) (Qiagen, catalog number: 74134 )
- RPE (RNA wash buffer) (Qiagen, catalog number: 74134 )
- RNeasyR Plus Mini Kit (Qiagen, catalog number: 74134 )
- Alternatively the Qiagen RNeasy Kit not including the gDNA separation columns may be used (Qiagen, catalog number: 75154 )
- RNase-free DNase Set (Qiagen, catalog number: 79254 )
- Buffers and separation columns (RNeasy Plus Mini Kit) (Qiagen, catalog number: 74134 )
- Ethanol (CH3CH2OH) (Merck, catalog number: 02851-1L )
- Ethanol 70% in H2O (see Recipes)
- RLT + β-ME or instead RLT-DTT buffer (see Recipes)
Equipment
- Adjustable Pipettes (Sartorius Biohit Proline France), or Micropipettes PIPETMAN L (GILSON, catalog number: 070053-070058 )
- Reagent Vessel (Sartorius, catalog number: 783500 )
- Surgical instruments (including a pair of scissors, two pair of fine scissors, and two tweezers) (Figure 1i-iv)
- Mice dissecting table and dissecting glass plate (Figure 1v).
- Precellys 24 System tissue homogenizer (Bertin Technologies, catalog number: P000669-PR240-A.0 )
- Precellys® 2 ml Hard Tissue homogenizing ceramic beads kit (CK28) (Bertin Instruments, catalog number: 10011151 , 230 Rockville, MD 20850 USA)
- 4 °C fridge and -20 °C freezer
- Centrifuge Eppendorf 5415R or 5424R (Eppendorf, catalog number: 5404000410 , Radius 8.3 cm)
- NanoDrop 1000 Spectrophotometer (Thermo Fisher Scientific, catalog number: ND-1000 ) (Schroeder et al., 2006)
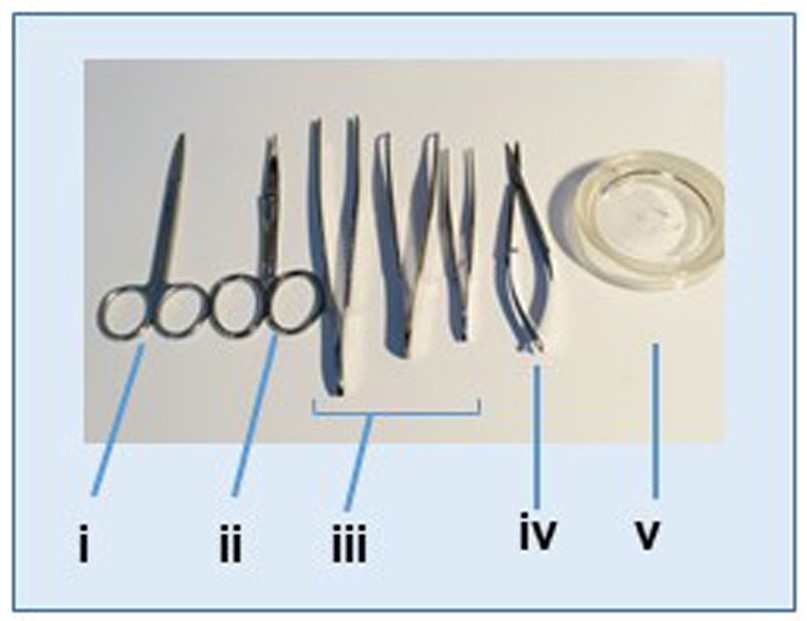
Figure 1. Instruments used for tissues dissection and lymph node isolation. i. Scissors; ii. Curved scissors; iii. Tweezers; iv. Fine scissors; v. Tissue dissection glass plate.
Procedure
文章信息
版权信息
© 2020 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Giraud, E. and Melanitou, E. (2020). RNA Extraction from Ears and Draining Lymph Nodes of Mice Infected with Leishmania amazonensis. Bio-protocol 10(11): e3633. DOI: 10.21769/BioProtoc.3633.
分类
免疫学 > 动物模型 > 小鼠
微生物学 > 微生物-宿主相互作用 > 体内实验模型 > 哺乳动物
分子生物学 > RNA > RNA 提取
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link












