- EN - English
- CN - 中文
Analysis of Generalized Fibrosis in Mouse Tissue Sections with Masson’s Trichrome Staining
Masson Trichrome染色法分析小鼠组织切片中的普遍纤维化
发布: 2020年05月20日第10卷第10期 DOI: 10.21769/BioProtoc.3629 浏览次数: 10365
评审: Alka MehraVasiliki KoliarakiAnonymous reviewer(s)
Abstract
Expansion of fibrous connective tissue and abnormal deposition of extracellular matrix (ECM) are at the basis of many fibrotic diseases. Fibrosis can occur in response to both physiological and pathological cues, including wound healing, tissue remodeling/repair and inflammation. Chronic fibrosis can lead to severe tissue damage, organ failure and death. Assessing the extent of organ fibrosis is crucial for accurate diagnosis of this condition. The use of Masson’s trichrome staining of tissue sections from skeletal muscle is a fast method for detection of morphological alterations indicative of a fibrotic phenotype in this organ. This staining method detects the extent of collagen fibers deposition and, because it employs the combination of three dyes, can also distinguish muscle fibers (red), from collagen (blue) and nuclei (black), simultaneously.
Keywords: Masson’s Trichrome (masson三色染色)Background
Fibrosis is the formation of excessive fibrous connective tissue in an organ as a result of chronic inflammation, tissue damage/remodeling (for instance, following chemical or radiation therapy), persistent infections, autoimmune disease, allergic responses and cancer. During this process excessive extracellular matrix (ECM) components, including collagens, are deposited and accumulate. If progressive, fibrosis becomes chronic, ultimately leading to organ failure and even death (Rockey et al., 2015). Several types of fibrotic diseases have been described in humans, many of which are of unknown etiology. The organs most commonly affected are the lungs, kidneys, liver, heart and skeletal muscle (Hinderer et al., 2019; Majo et al., 2019; Mahdy, 2019). Idiopathic pulmonary fibrosis, for instance, is a common, progressive and often fatal disease associated with scarring of the lung tissue that gradually looses the capacity to oxygenate, leading to the patient’s inability to breathe properly (Lederer et al., 2018).
A fibrotic process entails the activation of fibroblasts into myofibroblasts, cells that acquire a stellate or spindle-shape morphology, are motile and contractile, express α-smooth muscle actin, and secrete/remodel the ECM, altering the stiffness, morphology and composition of the tissue (Gattazzo et al., 2014; Kendall et al., 2014; Rockey et al., 2015). In the skeletal muscle under healthy conditions fibrosis occurs in the form of scar tissue during the healing process following muscle injury. This is usually associated with infiltration of inflammatory cells that induce satellite cells to proliferate and differentiate into new myotubes and myofibers while simultaneously the ECM undergoes remodeling. In disease conditions, such as muscular dystrophies or myopathies, progressive expansion of the connective tissue and abnormal deposition of ECM components, consequent to myofiber degeneration, ultimately results in full-blown fibrosis (Mahdy, 2019). We have recently discovered that deficiency of the sialic acid processing enzyme neuraminidase 1 (NEU1) in the mouse model of the rare pediatric lysosomal storage disease sialidosis, triggers a persistent expansion of the connective tissue, leading to generalized fibrosis in several organs including the liver, kidney, heart and skeletal muscle (Zanoteli et al., 2010; van de Vlekkert et al., 2019). Detection of the fibrotic disease was carried out using Masson’s trichrome staining of tissue sections from skeletal muscle, lung, heart, kidney and liver (Figure 1). This method is generally more accurate and informative than the standard hematoxilin & eosin staining because Masson’s trichrome staining not only maintains intact the overall morphology of the tissues, but also has the advantage of utilizing three dyes that allow for the identification of multiple tissue structures. In skeletal muscle the three dyes, Weigert’s iron hematoxylin, Biebrich scarlet and aniline blue, applied sequentially, allow to distinguish nuclei, muscle fibers and erythrocytes, and collagen fibers, respectively (Figure 1). This method is suitable for the assessment of the extent and distribution of fibrosis in a quantifiable manner, and can be successfully applied for the detection/diagnosis of fibrotic diseases.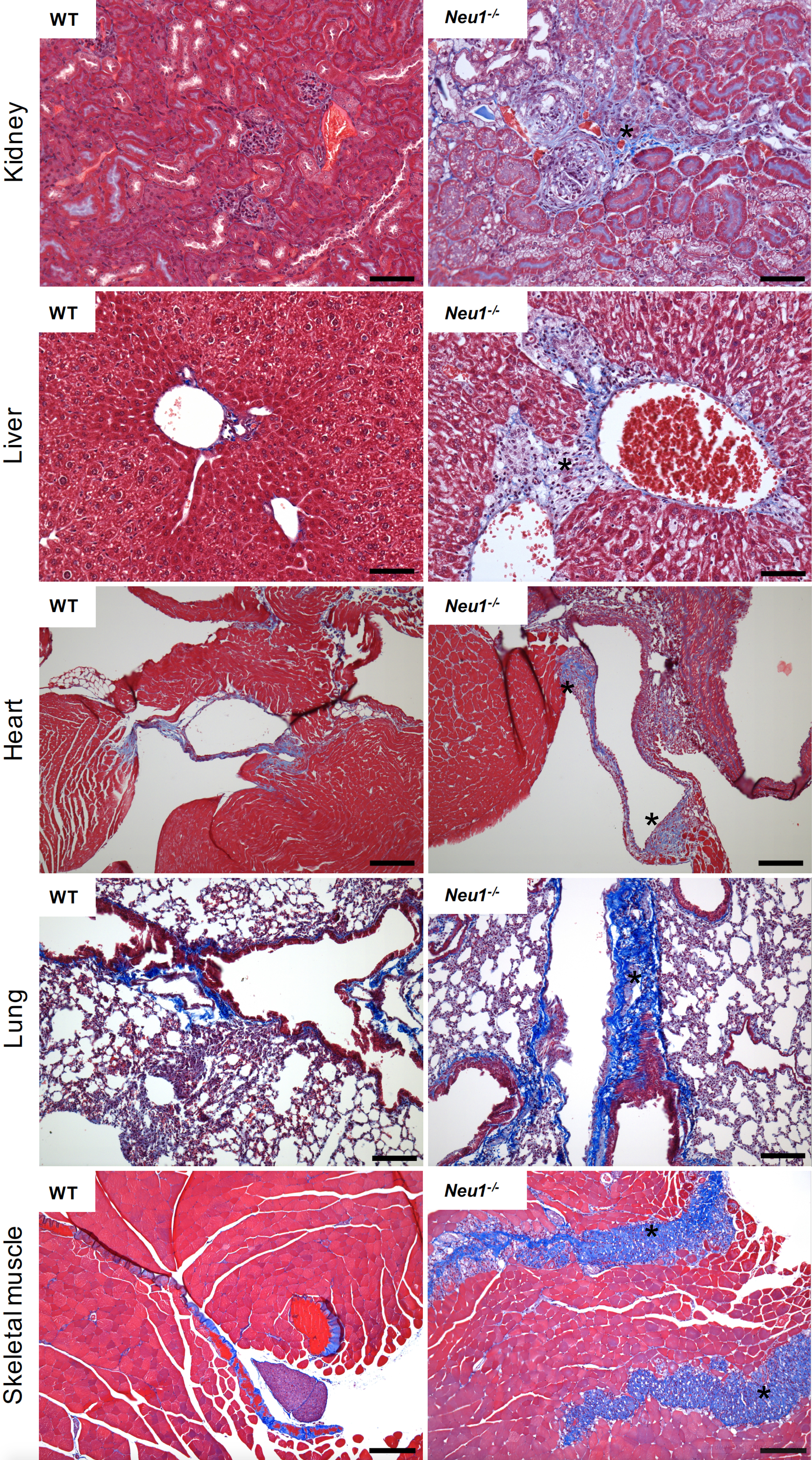
Figure 1. Masson’s Trichrome staining of multiple organs collected from the WT and Neu1–/– mice. Fibrotic regions in the Neu1–/– mouse are characterize by massive collagen deposition and therefore appear in blue (asterisks) (Images of WT and Neu1–/– kidney, liver and skeletal muscle are from van de Vlekkert et al., 2019). Blue = collagens; Red = erythrocytes and cytoplasm; Dark purple/black = nuclei. Scale bars: kidney and liver = 100 μm, heart, lung and skeletal muscle = 200 μm.
Materials and Reagents
Note: All reagents and materials should be kept at room temperature unless otherwise described. For the shelf life and storage temperature of reagents we refer to the manufacturer’s instructions.
- Lab coat and gloves
- BD tuberculin syringes (Fisher, BD Biosciences, catalog number: 14-826-88 or BD309626)
- Exel International 10 to 12cc syringes (Fisher, catalog number: 14-841-54)
- Needles (25 G x 5/8 inch): BD General use and precisionGlide hypodermic needle (Fisher, BD Biosciences, catalog number: 14-826AA or BD305122)
- Aluminum dissecting pan with vinyl dissecting pad (Carolina, catalog number: 629004)
- Light duty paper wipes (Georgia Pacific, Brawny, catalog number: 29221)
- 50 ml Falcon conical centrifuge tubes (Fisher Scientific, Corning, catalog number: 14-432-22)
- Scientific slide holder (Starplex Scientific, catalog number: V302SH)
- Tissue-Tek® base molds, stainless steel (Sakura, catalog number 4217)
- Tissue-Tek® Uni-cassette, white (Sakura, catalog number: 4170)
- Superfrost Plus Microscope slides, white (Fisher Scientific, Fisherbrand, catalog number: 12-550-15)
- Microscope cover glass (24x50-1.5) (Fisherbrand, Fisher Scientific, catalog number: 12-544-E)
- Microscope slide box (Fisherbrand, Fisher Scientific, catalog number: 03-448)
- Adult control and experimental mice (e.g., WT and Neu1-/-)
Notes:- The generation and initial characterization of the Neu1-/- is described in de Geest et al., 2002. WT and Neu1-/- mice are maintained in house.
- The gastrocnemius muscle, liver, kidneys, lung and heart from WT and Neu1-/- mice (FVB/NJ) at 4 months of age are used for the purpose of this protocol. Any background, gender, age or tissue type (paraffin or frozen) can be used for Masson’s staining.
- Avertin (tribromoethanol; 12.5 mg/ml); provided by ARC veterinary services at SJCRH
- Dulbecco’s Phosphate Buffered Saline (DPBS) (Corning, catalog number: 21-031-CV)
- Advantus T-pins (Fisher scientific, catalog number: S174301)
- Prefilled container, 10% Neutral Buffered Formalin, 180 ml (Thermo Scientific, catalog number: 591801)
- Paraffin (Thermo Fisher Scientific, catalog number: 8337)
- Histoprep Xylene (Fisherbrand, catalog number: HC7001GAL)
- Ethanol 200 proof (Pharmco by Greenfield Global, catalog number: 111000200)
- Ethanol 190 proof (Pharmco by Greenfield Global, catalog number: 111000190)
- Ethanol 140 proof (Pharmco by Greenfield Global, catalog number: 111000140)
- Bouin’s Fixative (Polysciences, catalog number: 16045-1)
- Hematoxylin (Polysciences, catalog number: 02749-25)
- 29% ferric chloride (Rowley Biochemical, catalog number: SO-125A)
- Hydrochloric acid (Fisher Scientific, catalog number: A144S-500)
- Biebrich scarlet, C.I.26905 (Polysciences, catalog number: 0336-100)
- Acid fuchsin, C.I. 42685 (Polysciences, catalog number: 24991)
- Acetic acid, glacial (Fisher Scientific, catalog number: A38S-500)
- Phosphotungstic acid (Sigma, catalog number: P4006-25)
- Phosphomolybdic acid (Polysciences, catalog number: 01021-25)
- Aniline blue, C.I. 42755 (Polysciences, catalog number: 02570-25)
- Cytoseal XYL (Thermo Fisher Scientific, catalog number: 8312-4)
- Weigert’s iron hematoxylin (see Recipes)
Solution A
Solution B - Biebrich scarlet-acid fuchsin solution (see Recipes)
- Phosphotungstic/phophomolybdic acid (see Recipes)
- Aniline blue (see Recipes)
- 1% acetic acid (see Recipes)
Equipment
- Dissection tools: sterilized scissors and forceps (Roboz, catalog numbers: RS-5983, RS-5877, RS-5358, RS-5135)
- Isotemp Incubator (Fisherbrand, Fisher Scientific, catalog number: 15-103-0513)
- Tissue processor Excelsior AS (Thermo Fisher Scientific, catalog number: A82300001).
Note: For manufacturer instructions go to: https://assets.fishersci.com/TFS-Assets/APD/manuals/A82310100_05%20-%20Operator%20Guide%20-%20Hi%20Res.pdf - Heated paraffin embedding station (HistoCore Arcadia H, Leica Biosystems)
- Cold plate (HistoCore Arcadia C, Leica Biosystems)
- Shandon Para Trimmer (Thermo Fisher Scientific, catalog number: B3120205)
- Microtome fully automated rotary (Leica Biosystems, catalog number: RM2255).
Note: For manufacturer instructions go to: https://drp8p5tqcb2p5.cloudfront.net/fileadmin/downloads_lbs/Leica%20RM2255/User%20Manuals/Leica_RM2255_IFU_2v3J_en.pdf - American Painter brush, ½ inch (Loew-Cornell, series 4550 Wash)
- Water bath for paraffin sections (Leica Biosystems, model: Leica HI1210)
- Bead bath (Precision Scientific, model 83, catalog number: 66551)
- Lab Armor Beads (Thermo Fisher Scientific, catalog number: A1254301)
- Glass Coplin slide staining jars (Thermo Fisher Scientific, catalog number: E94)
- General purpose themometer (Fisherbrand, catalog number: 13-201-644)
- Fume hood present in the laboratory
- Gemini AS automated slide stainer (Thermo Fisher Scientific, catalog number: A81500002).
Note: For manufacturer instructions go to: https://assets.thermofisher.com/TFS-Assets/APD/manuals/Gemini%20AS%20Operator%20Guide.pdf - Light microscope with camera (Leica, catalog numbers: DM2500 and DF450)
Software
- Imaging Software. Leica Application Suite (LAS) Version 4.9.0 (Build:129) (Leica Microsystems, CMS GmbH)
Procedure
文章信息
版权信息
© 2020 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- van de Vlekkert, D., Machado, E. and d'Azzo, A. (2020). Analysis of Generalized Fibrosis in Mouse Tissue Sections with Masson’s Trichrome Staining. Bio-protocol 10(10): e3629. DOI: 10.21769/BioProtoc.3629.
- van de Vlekkert, D., Demmers, J., Nguyen, X. X., Campos, Y., Machado, E., Annunziata, I., Hu, H., Gomero, E., Qiu, X., Bongiovanni, A., Feghali-Bostwick, C. A. and d'Azzo, A. (2019). Excessive exosome release is the pathogenic pathway linking a lysosomal deficiency to generalized fibrosis. Sci Adv 5(7): eaav3270.
分类
细胞生物学 > 组织分析 > 组织染色
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link