- EN - English
- CN - 中文
Permethylation and Microfractionation of Sulfated Glycans for MS Analysis
硫酸多糖的全甲基化和微萃取质谱分析
发布: 2020年05月20日第10卷第10期 DOI: 10.21769/BioProtoc.3617 浏览次数: 4655
评审: Manjula MummadisettiAnonymous reviewer(s)
Abstract
Sulfated glycans are barely detectable in routine mass spectrometry (MS)-based glycomic analysis due to ion suppression by the significantly more abundant neutral glycans in the positive ion mode, and sialylated non-sulfated glycans in the negative ion mode, respectively. Nevertheless, the negative charge imparted by sulfate can be advantageous for selective detection in the negative ion mode if the sialic acids can first be neutralized. This is most conveniently achieved by a concerted sample preparation workflow in which permethylation is followed by solid phase fractionation to isolate the sulfated glycans prior to MS analysis. Importantly, we demonstrated that conventional NaOH/DMSO slurry permethylation method can retain the sulfates. Instead of extracting permethylated glycans into chloroform for sample clean-up, reverse phase C18 cartridge coupled with self-packed amine-tip or mixed mode weak anion exchange cartridge can be utilized to obtain in good yield the non-sulfated, mono-sulfated, and multiply sulfated permethylated glycans in separate fractions for sulfoglycomic analysis.
Keywords: Permethylated sulfated glycans (全甲基化硫酸多糖)Background
Sulfated glycans carrying sulfo sialyl LeX glycotope are ligands of L-selectin involved in lymphocyte homing in physiological and inflammatory states (Rosen, 2004; Kawashima, 2006). Many glycan array studies also showed that sulfated glycotopes are better ligands of several galectins (Ideo et al., 2002) and Siglecs (Bochner et al., 2005). However, there is very limited knowledge of endogenous sulfated glycans expressed on different types of mammalian cells, tissues or organism due to their low abundance and availability of few well-defined antibodies. Advanced mass spectrometry (MS)-based analysis is the only viable technique that would afford the requisite high sensitivity and accuracy for a meaningful probing of the cellular sulfoglycome.
Sulfated glycans are not readily detected in the conventional MS-based glycomic profiling. For analysis of non-derivatized, native sulfated glycans in the negative ion mode, the main problem is that sialylated glycans also carry negative charges and these are usually more abundant. Removing the sialic acids by treatment with sialidase is commonly applied (Hernandez Mir et al., 2009), but information on the sulfated sialylated glycans, which maybe the true physiological ligands for endogenous lectins is lost. For permethylated glycans, the advantages are neutralization of the negative charge of sialic acid and more reliable, sequence informative fragment ions can be obtained by MS/MS analyses. A precondition though is that the widely used sample clean-up method following permethylation, such as extraction by chloroform/water partition, which would result in loss of permethylated sulfated glycans into the aqueous layer, should be avoided (Morelle and Michalski, 2007; Kumagai et al., 2013).
We have shown that the conventional NaOH/DMSO slurry permethylation can retain the sulfates, and reverse phase (C18) solid phase extraction cartridge can be used to replace chloroform extraction (Yu et al., 2009; Khoo and Yu, 2010). Both permethylated non-sulfated and sulfated glycans are retained, extensively washed, and then eluted without introducing salts. MALDI-MS screening of permethylated glycans in the negative ion mode gives a quick survey for the presence of sulfated glycans. Instead of using 2,5 dihydroxybenzoic acid (DHB), which is a common matrix for glycan analysis in the positive ion mode (Harvey, 1993), we found that 3,4-diaminobenzophenone (DABP) afforded a better sensitivity for detecting permethylated sulfated glycans in the negative ion mode. Non-sulfated, mono-sulfated, di-sulfated permethylated glycans can be further fractionated by using a self-packed amine tip. Alternatively, a mixed mode weak anion exchange (MAX) cartridge containing both reverse phase and weak anion exchange properties can be used to replace the two-step process of cleaning and fractionation (Cheng et al., 2013; Cheng et al., 2015; Hsiao et al., 2017). The developed workflow for sulfoglycomics (Figure 1) has been successfully applied to many different biological samples such as lymph nodes from different glycosyltransferase/sulfotransferase knockout mice (Mitoma et al., 2007; Patnode et al., 2013), ovarian cancer (Shibata et al., 2012; Yu et al., 2013), colon cancer (Yu et al., 2018), immune cells (Patnode et al., 2013; Wang et al., 2013). Collectively, it showed that sulfated N- and O-glycans have a wider and ubiquitous occurrence than previously appreciated.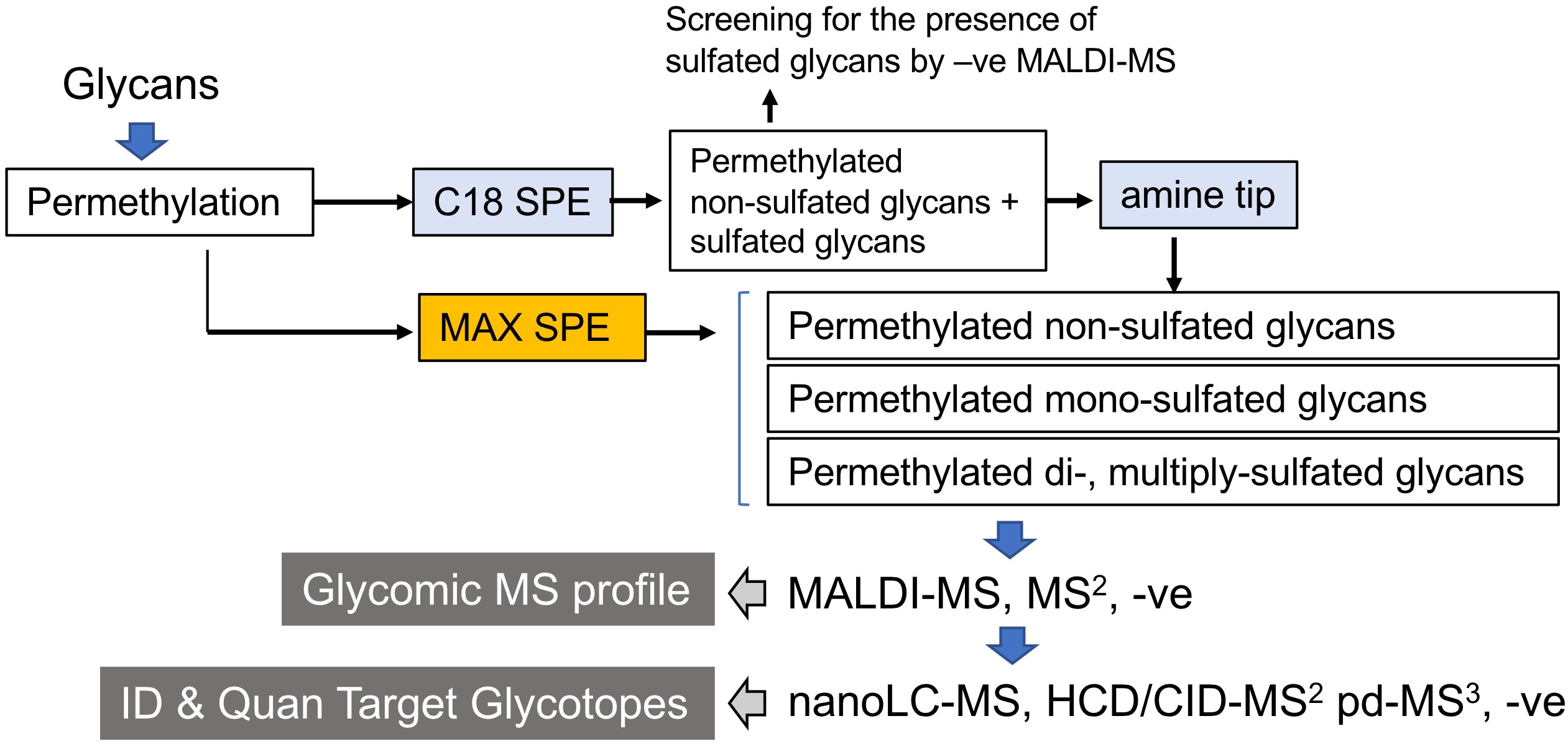
Figure 1. Schematic workflow for MS-based sulfoglycomic analyses. The key steps are reverse phase C18 which retains all permethylated glycans, and further fractionation of sulfated permethylated glycans from non-sulfated ones by amine beads. Alternatively, a mixed mode anion exchange (MAX) SPE is equally effective. MALDI-MS screening in the negative ion mode affords a quick profile of the sulfoglycome. This protocol is applicable to all commonly found sulfated N-glycans and O-glycans, irrespective of their reducing end status. The procedure for additional nanoLC-nanoESI-MS/MS analyses was described in another paper entitled “Negative Ion Mode nanoLC-ESI-MS/MS Analyses of Permethylated Sulfated Glycans” in Bio-protocol (Yu et al., 2020). SPE, solid phase extraction.
Materials and Reagents
- C18 cartridge (Waters, Sep-Pak, catalog number: WAT091139 )
- ZipTipC18 (Merck Millipore, catalog number: ZTC18S096 )
- NUCLEOSIL® 100-5 NH2 (MACHEREY-NAGEL, Nucleosil, catalog number: 712200.1 0)
- Disposable 20 ml Syringe (BD, catalog number: 300296 )
- Filter paper
- Oasis MAX 3 cc Vac Cartridge (Waters, Oasis, catalog number: 186000367 )
- Several clear chemical glass bottles, 250 ml (PYREX®, catalog number: 1515-06D ), 500 ml (PYREX®, catalog number: 1515-08D )
- PYREX® 13 x 100 mm round bottom glass tubes (Corning, catalog number: 99449-13 )
- Corning® Reusable Phenolic GPI 13-415 screw cap with PTFE Liner (Corning, catalog number: 9998-13 )
- Glass Pasteur pipette (Corning, catalog number: 7095B-9 ) and plastic pipette tips
- pH-indicator strips pH 0-14 Universal indicator (Merck Millipore, catalog number: 1.09535.0001 )
- 1.5 ml Eppendorf safe-lock tubes (Eppendorf, catalog number: 00 30120086 )
- Sodium hydroxide (NaOH) (Merck Millipore, catalog number: 106498 )
- Dimethyl sulfoxide (DMSO) (Merck Millipore, catalog number: 1029310161 )
- Methyl iodide (stabilized with silver) (Merck Millipore, catalog number: 806064 )
- 100% ethanoic acid (glacial) (Merck Millipore)
- Acetonitrile (ACN) (VWR chemicals, catalog number: 20060.32 0)
- Methanol (VWR chemicals, catalog number: BDH1135-1LP )
- 3,4-diaminobenzophenone (DABP) (Acros OrganicsTM, catalog number: 184800250 )
- Ammonium acetate (NH4OAc) (Sigma Aldrich, catalog number: 5.43834 )
- Trifluoroacetic acid (TFA) (Sigma-Aldrich, catalog number: 302031-100m ), store in 4 °C
- Formic acid (FA) (Merck Millipore, catalog number: 5.438040.100 )
- 2.5% ACN (200 ml) (see Recipes)
- 10% ACN (200 ml) (see Recipes)
- 25% ACN (200 ml) (see Recipes)
- 50% ACN (200 ml) (see Recipes)
- 0.1% TFA (50 ml) (see Recipes)
- 75% ACN, 0.1% TFA (50 ml) (see Recipes)
- 95% ACN (50 ml) (see Recipes)
- 95% ACN, 0.1% FA (50 ml) (see Recipes)
- 50% ACN, 0.1% FA (50 ml) (see Recipes)
- 1 M ammonium acetate (NH4OAc) (see Recipes)
- 50% ACN, 2.5 mM NH4OAc (50 ml) (see Recipes)
- 50% ACN, 10 mM NH4OAc (50 ml) (see Recipes)
- 100 mM NH4OAc (200 ml) (see Recipes)
- 80% ACN, 1 mM NH4OAc (200 ml) (see Recipes)
- 60% ACN, 20% methanol, 100 mM NH4OAc (200 ml) (see Recipes)
Equipment
- Ceramic mortar and pestle
- Laboratory fume hood
- 50 °C heater
- Vortex-Genie® 2 (Scientific Industries) coupled with 9-16 mm foam inserts (Scientific Industries, model: 503-0278-00 )
- Savant SpeedVac® concentrator (Thermo Scientific, model: ISS110 ) with rotor (Thermo Scientific, catalog number: RH20-12 )
- MALDI-TOFTOF 4700 Proteomics Analyzer or any equivalent MALDI-TOFTOF series (Applied Biosystems, Sciex) mass spectrometer
Software
- Data Explorer version 4.5 (Applied Biosystems)
Procedure
文章信息
版权信息
© 2020 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Yu, S., Snovida, S. and Khoo, K. (2020). Permethylation and Microfractionation of Sulfated Glycans for MS Analysis. Bio-protocol 10(10): e3617. DOI: 10.21769/BioProtoc.3617.
- Yu, S. Y., Hsiao, C. T., Izawa, M., Yusa, A., Ishida, H., Nakamura, S., Yagi, H., Kannagi, R. and Khoo, K. H. (2018). Distinct substrate specificities of human GlcNAc-6-sulfotransferases revealed by mass spectrometry-based sulfoglycomic analysis. J Biol Chem 293(39): 15163-15177.
分类
生物化学 > 糖类 > 多糖
生物化学 > 糖类 > 糖蛋白
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link