- EN - English
- CN - 中文
In vivo Quantification of Alkanes in Escherichia coli
大肠杆菌中脂肪醛去甲酰加氧酶产生烷烃的体内量化
发布: 2020年04月20日第10卷第8期 DOI: 10.21769/BioProtoc.3593 浏览次数: 4769
评审: Juan Facundo Rodriguez AyalaJose Antonio Reyes-DariasAnonymous reviewer(s)
Abstract
Microbial production of alkanes employing synthetic biology tools has gained tremendous attention owing to the high energy density and similarity of alkanes to existing petroleum fuels. One of the most commonly studied pathways includes the production of alkanes by AAR (acyl-ACP (acyl carrier protein) reductase)-ADO (aldehyde deformylating oxygenase) pathway. Here, the intermediates of fatty acid synthesis pathway are used as substrate by the AAR enzyme to make fatty aldehyde, which is then deformylated by ADO to make linear chain alkane. However, the variation in substrate availability to the first enzyme of the pathway, i.e., AAR, via fatty acid synthesis pathway and low turnover of the ADO enzyme make calculation of yields and titers under in vivo conditions extremely difficult. In vivo assay employing external addition of defined substrates for ADO enzyme into the medium helps to monitor the influx of substrate hence providing a more accurate measurement of the product yields. In this protocol, we include a detailed guide for implementing the in vivo assay for monitoring alkane production in E. coli.
Background
Research on alkane production using engineered microbes has gained significant popularity as it provides an attractive alternative to reduce dependence on fossil fuels while mitigating the climate change effects (Lee et al., 2008; Knothe, 2010; Lu, 2010; Schirmer et al., 2010; Tan et al., 2011). Various pathways have been uncovered or artificially assembled for the production of alka(e)nes in microbes (Schirmer et al., 2010; Mendez-Perez et al., 2011; Rude et al., 2011; Akhtar et al., 2013; Howard et al., 2013; Rui et al., 2014). However the highest reported titres for alkane production so far have been in E. coli using the AAR-ADO pathway (Figure 1) (Fatma et al., 2018). AAR catalyzes the reduction of fatty acyl-ACP or fatty acyl-CoA into fatty aldehydes using NADPH which is subsequently converted into alkanes by ADO (Marsh et al., 2013). E. coli is the most widely used host for heterologous production of biofuel candidates due to a broader knowledge of its cellular metabolic network as compared to other hosts. Heterologous co-expression of cyanobacterial AAR and ADO in E. coli results in production and secretion of alkanes (Schirmer et al., 2010). However, quantification of alkanes by the ADO enzyme and determination of the in vivo efficacy of the ADO enzyme cannot be determined due to the variation in the substrate availability by the first enzyme AAR of the pathway. Reports on differences in the solubility of AAR and hence its activity (Kudo et al., 2016) indicate that using AAR as a source of substrate for measuring in vivo activity of ADO is not a suitable approach. Hence, we developed an in vivo enzyme assay, which involves addition of the substrate aldehyde exogenously to the medium that is taken up by the growing cells and gets converted to alkane by the heterologously expressed intracellular ADO enzyme, thus giving a more reliable and accurate measurement of its in vivo efficacy.
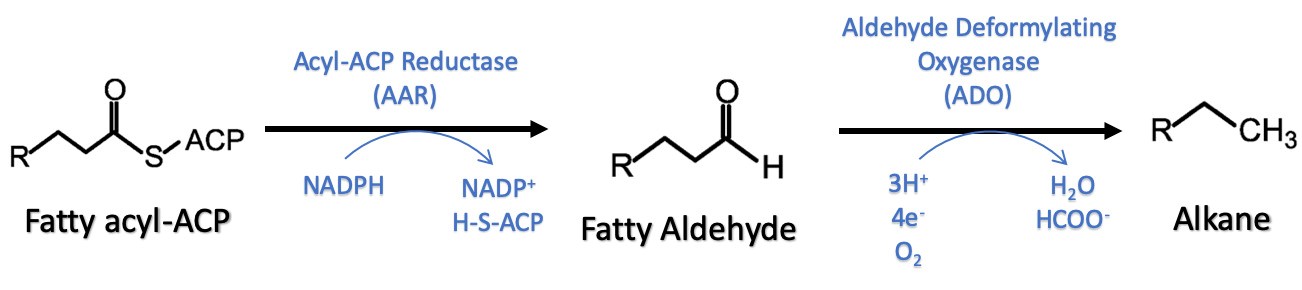
Figure 1. Schematic representation of AAR-ADO pathway for alkane production
Materials and Reagents
- 2 ml micro-centrifuge tubes (Tarson, catalog number: 500020 )
- Culture tubes (55 ml, Borosil, catalog number: 9820U08 )
- GC vials (Agilent, catalog number: 5190-2280 )
- Parafilm (Sigma, catalog number: P7793 )
- 0.2 μm filter (mdi Membrane Technologies, catalog number: SYNN0301MNXX104 )
- E. coli DH5α strain overexpressing ADO enzyme cloned in pQE30 vector (strain source: Invitrogen, vector source: Qiagen)
- Hexadecanal (TCI America, catalog number: H1296 )
- Octadecene (TCI America, catalog number: S0348 )
- Sodium phosphate dibasic (Na2HPO4) (Merck, catalog number: 255793 )
- Potassium dihydrogen phosphate (KH2PO4) (Sigma, catalog number: P5655 )
- Sodium chloride (NaCl) (Sigma, catalog number: S9888 )
- Ammonium chloride (NH4Cl) (Sigma, catalog number: 213330 )
- Magnesium sulphate heptahydrate (MgSO4·7H2O) (Sigma, catalog number: 230391 )
- Calcium chloride (CaCl2) (Sigma, catalog number: C4901 )
- Ferric chloride hexahydrate (FeCl3·6H2O) (Sigma, catalog number: 236489 )
- Zinc chloride (ZnCl2) (Sigma, catalog number: 746355 )
- Sodium molybdate dihydrate (Na2MoO4·2H2O) (Sigma, catalog number: 331058 )
- Copper sulphate (CuSO4) (Sigma, catalog number: C1297 )
- Boric acid (H3BO3) (Sigma, catalog number: B7901 )
- Thiamine hydrochloride (Sigma, catalog number: T4625 )
- Glucose (Sigma, catalog number: G8270 )
- Bis Tris (Amresco, catalog number: 0715-250G )
- Triton X-100 (Amresco, catalog number: M143-1L )
- Ethyl acetate (Merck, catalog number: 1096232500 )
- IPTG (Sigma, catalog number: I6758 )
- LB broth (HiMedia, catalog number: M1245 )
- Ampicillin (Sigma, catalog number: A9393 )
- M9 salts (see Recipes)
- M9 modified medium (see Recipes)
Equipment
- Table top centrifuge (Eppendorf, models: 5418R and 5810R)
- Incubator shaker (Kuhner, model: ISF-1 )
- GC FID (Agilent, model: 7890 A System , equipped with HP-5 column with catalog no. 19091J-413 )
- Spectrophotometer (GE Healthcare, model: UltrospecTM 2100)
- Vortex (Sigma, catalog number: Z258423-1EA )
- Autoclave (Natsteel, model: 24 SR )
Procedure
文章信息
版权信息
© 2020 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Shakeel, T., Fatma, Z. and Yazdani, S. S. (2020). In vivo Quantification of Alkanes in Escherichia coli. Bio-protocol 10(8): e3593. DOI: 10.21769/BioProtoc.3593.
- Shakeel, T., Gupta, M., Fatma, Z., Kumar, R., Kumar, R., Singh, R., Sharma, M., Jade, D., Gupta, D., Fatma, T., Yazdani, S. S. (2018). A consensus-guided approach yields a heat-stable alkane-producing enzyme and identifies residues promoting thermostability. J Biol Chem 293(24): 9148-9161.
分类
微生物学 > 异源表达系统 > 大肠杆菌
生物化学 > 其它化合物 > 烷烃
生物化学 > 蛋白质 > 活性
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link