- EN - English
- CN - 中文
Minimally Invasive Oral Surgery Induction of the FRICT-ION Chronic Neuropathic Pain Model
微创口腔手术诱导FRICT-ION慢性神经病理性疼痛模型的建立
(*contributed equally to this work) 发布: 2020年04月20日第10卷第8期 DOI: 10.21769/BioProtoc.3591 浏览次数: 5106
评审: Alessandro DidonnaHeather L RossiAnonymous reviewer(s)

相关实验方案

基于 rAAV-α-Syn 与 α-Syn 预成纤维共同构建的帕金森病一体化小鼠模型
Santhosh Kumar Subramanya [...] Poonam Thakur
2025年12月05日 1658 阅读
Abstract
An easily induced preclinical trigeminal neuropathic nerve injury model is described here for the study of chronic pain, the model acronym FRICT-ION (Foramen Rotundum Inflammatory Constriction Trigeminal InfraOrbital Nerve). In patients, neuropathic pain is thought to be related to vascular alignment or multiple sclerosis along this small trigeminal nerve branch (V2) innervating the maxillary teeth and middle third of the face. With no detectable outward physical signs, the FRICT-ION model is ideal for blinded studies. The acronym FRICT-ION applied relates to the persistence of the trigeminal neuropathic pain model likely due to sliding irritation with normal chewing in the mice. A step-by-step method to induce the mild chronic rodent neuropathic pain model is described here. The surgery is performed orally through a tiny surgical slit inside the cheek crease to align a chromic gut suture irritant along the nerve as it passes into the skull. The model allows testing of non-evoked subjective measures and evoked quantitative mechanical hypersensitivity (allodynia) testing with von Frey filaments through at least 10-14 weeks (100 days). Anxiety and depression behaviors develop within 3-6 weeks relevant to the affective component of chronic pain. While many pain drugs have failed based on testing performed in the acute animal models available, the more stable and easily replicated trigeminal inflammatory compression model is the better suited for understanding both mechanistic and affective components of nerve injury-induced chronic neuropathic pain states as well as the more ideal for preclinical trials of novel non-opioid pain relief therapies.
Keywords: Chronic pain (慢性疼痛)Background
Damaged peripheral nerves cause persisting overactivation referred to as “neuropathic pain”. A serious consequence of persisting nerve injury pain is the transition to chronic pain. Neuropathic pain is the result of changes in the signaling molecules in the peripheral nerve cells that eventually cause central sensitization and changes in the brain’s pain and affective circuitry. A major obstacle to better understanding of pathophysiological mechanisms of chronic neuropathic pain and development of effective therapeutics is lack of available experimental animal models that mimic established chronic pain. To emulate chronic orofacial neuropathic pain, the FRICT-ION (Foramen Rotundum Inflammatory Constriction of Trigeminal InfraOrbital Nerve) nerve injury model was developed in mice to study behavioral, pharmacological, cellular, and molecular mechanisms persisting during chronic neuropathic pain.
While many nerve injury models have been developed that allow study of nociceptive mechanisms and pain-related behaviors in the short term, the resilience of natural healing processes typically reverses nerve injury models within 3-4 weeks in neuropathic pain models. The method described below is one of the few models that allow long-term studies suitable for better understanding of clinical pain states and for testing experimental therapies. Several innate differences in the trigeminal nerve are likely responsible for the chronic persistence of the neuropathic model. The great importance of the trigeminal nerve to survival is postulated to be one characteristic responsible for the ease in producing persistence of trigeminal nerve injury pain and its effects on psychological well-being (Carlson, 2007). Anatomical proximity to the brain and the unique circuitry of the trigeminal system with one less synapse forming more direct connectivity with the limbic circuitry are also likely responsible for the intensity and persistence of neuropathic pain associated with the trigeminal nerve (Rodriguez et al., 2017).
Previous successful chronic trigeminal pain models described have been induced by loose chromic gut suture tie of the infraorbital nerve (ION) in rats (CCI-ION) behind the eye (Vos et al., 1994; Kniffin et al., 2015) or on the snout in rats (dIoN-CCI) and mice (DIONI) (Ding et al., 2017; Hardt et al., 2019) (Figure 1). Alternatively, the intraoral approach has also been used to induce the CCI-ION model in rats (Immamura et al., 1997). We previously introduced a trigeminal nerve hypersensitivity model induced by sliding the irritative chromic gut suture into the ION fissure behind the eye in mice creating a trigeminal inflammatory compression (TIC) (Figure 1) (Ma et al., 2012 and 2015; Lyons et al., 2015 and 2018). The surgical approach to the ION behind the eye is technically challenging and the vasculature prone to bleeding, often severely. After induction of trigeminal nerve injury models, mechanical hypersensitivity develops within a week and persists indefinitely in the ION’s whisker pad receptive field. After trigeminal nerve injury, cognitive deficits are reported within 3 weeks in rats and anxiety- and depression-related symptoms emerge in subsequent weeks after injury (>6 weeks) in rodents indicating brain origin and potential circuitry neuroplasticity within brain regions are responsible for these behaviors accompanying chronic pain (Yalcin et al., 2011 and 2014; Kniffin et al., 2015; Lyons et al., 2015 and 2018). Estimates are that in weeks 6-8 post-surgery, mice have experienced pain equivalent to 6-8 human years and persistence of models in this time frame can easily be considered chronic (Dutta and Sengupta, 2016; Hannaman et al., 2016). The interplay between psychologic and physical functioning is a particular consideration in patients with orofacial pain (Carlson, 2007).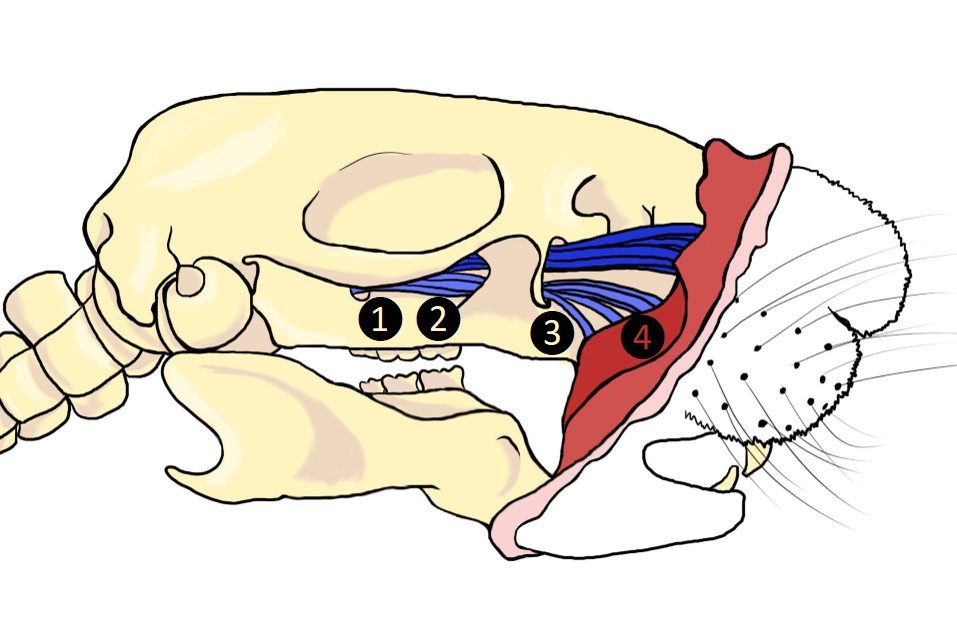
Figure 1. Surgical sites utilized in trigeminal neuropathic pain models. 1. TIC, 2. CCI-ION, 3. FRICT-ION, 4. dIoN-CCI and DIONI.
Better models for the study of chronic neuropathic pain are needed to discover more effective treatments since the current treatment of choice, microvascular decompression surgery, in patients with chronic trigeminal nerve pain or multiple sclerosis pain wanes over time with reoccurrence typically at 3 years (Xia et al., 2014). The pain-free rate at 5 and 10 years decreases to 61% and 44% after decompression surgery to place a Teflon spacer and after stereotaxic radioablation is only 47%, and 27%, respectively (Wang et al., 2018). Teflon granulomas are reported in 5.6% of patients after microvascular decompression (Chen et al., 2000).
The FRICT-ION method provides a simpler intraoral approach (Figure 1). The benefits of using the mouse FRICT-ION model of trigeminal nerve injury for study of chronic neuropathic pain in general are many. The model induces long-term chronic neuropathic pain with no other apparent side effects or outward physical indication. The mouse experiences no health or weight gain issues. The method exposes the rodent to only minutes of isoflurane anesthesia but sensitivity in the receptive field on the whiskerpad persists indefinitely. If done correctly, the minimally invasive surgery has little or no bleeding. The model is quickly and easily mastered following the instructions provided below.
Surgical Induction of the FRICT-ION Model
We induce the model in either BALBc or C57Bl/6 mice (20 to 25 g; 8-10 weeks; Harlan Laboratories, Indianapolis, IN). We have performed the procedure in older mice with no complications. The surgery is performed open mouth through a tiny slit to align chromic gut suture along the trigeminal nerve. The constant but minimal nerve irritation likely produced with chewing is similar to one known cause of trigeminal neuropathic pain, irritation by proximity of the nerve to pulsating brainstem vasculature which is relieved by insertion of a Teflon sheet (Dandy, 1934; Xia et al., 2014). The extended duration of orofacial neuropathic pain models provides an optimal platform for preclinical testing of potential therapeutics since mechanical and cold sensitivities, anxiety, and depression-like behaviors are measurable.
The model illustrated here is induced by inserting 3 mm of chromic gut suture intraorally into the tight space where the infraorbital nerve (ION) passes through the bony infraorbital foramen. Similar to nerve biopsies from patients (Weis et al., 2012), the TIC model we reported previously using the intraorbital surgical approach did not cause the axonal degeneration seen in tied or cut nerve models (Ma et al., 2012). As a result, the TIC model induced hypersensitivities similar to those in the human condition, i.e., mechanical and cold allodynia, but not heat hypersensitivity, along with anxiety and depression. Though not tested as yet, we are hopeful that these results will be duplicated using the intraoral approach to induce the FRICT-ION model. The model is illustrated here induced on the right side of the mice in all figures. The model has been induced in both males and females, BALBc and C57Bl/6, and while not yet tested by our lab, likely could also be done in rats. The intraoral approach has been used previously in rats to induce the nerve tied CCI-ION model (Immamura et al., 1997).
Materials and Reagents
- 0.2 cm diameter corroborated evac tubing (Patterson Veterinary, catalog number: 07-8914311 )
- 1 cm diameter Tygon tube (Fisher Scientific, catalog number: 14-171-104 )
- Scavenger filter of the anesthesia unit (Patterson Scientific WAG unit, catalog number: 78909457 )
- Stabilization/tension ties constructed from rubber bands and surgical silk (shopmedvet.com, 5-0 silk; 100M cassette SKU: SLK50)
- Embroidery floss (DMC, catalog number: 117S-3380 )
- Binder clip, ACCO 2” (Staples, catalog number: 72100 )
- Cotton-tipped applicators (shopmedvet.com, 6”, non-sterile, SKU: CTA6)
- Gloves
- BALBc or C57Bl/6 mice (20 to 25 g; 8-10 weeks; Harlan Laboratories, Indianapolis, IN)
- 70% ethanol (local source)
- Sucrose
Equipment
- 4-0 chromic gut suture (cut into 3 mm lengths) (Ethicon, catalog number: 635H )
- Forceps (Dumont No. 5, Fine Science Tools, catalog number: 11251-10 ; Graefe Angled Serrated, Fine Science Tools, catalog number: 11049-10 )
- No. 11 scalpel blades (Feather/Electron Microscopy #11, catalog number: 72044-11 , VWR, catalog number: 102097-822 )
- Scalpel handle #3 (Walter Stern 320-051) (VWR, catalog number: 25607-947 )
- Bead sterilizer (Germinator 500 Glass Bead Sterilizer, Cell Point Scientific 5-1450) (VWR, catalog number: 101326-488 )
- Heating pad/recovery station for mice (HTP-1500 Adroit Veterinary Heat Pump and Soft Temp Veterinary Heat Pad V016) (Patterson Veterinary Supply, catalog numbers: 78910655 and 78910866 )
- Surgery setup (see Procedure section below)
- Dissecting scope (Optika SZN-10 Trinocular Stereo Zoom Microscope on Hinged Overhanging Stand, New York Microscope Company, catalog number: OPSZN-10 )
- Isoflurane anesthesia machine [Tec 3 EX Isoflurane Standard Fill Vaporizer, Versa II Anesthesia system, with Mapleson-D Non-Rebreathing (NRB) System] (Patterson Veterinary Supply, catalog numbers: 78703592 , 78910545 with 78909636 )
- Base Plate, S&T Ball Chain Retraction (Fine Science Tools, catalog number: 18100-00 )
- Fiber Optic Light (Dolan-Jenner Fiber-lite Illumination System 181-1) (VWR, catalog number: 41446-062 )
- Von Frey Filaments (Semmes-Weinstein, 20 monofilaments, BioSeb, bioseb.com)
- Autoclave
Software
- Ethovision XT8 videotracking system software (Noldus Information Technology, Leesburg, VA, USA) for analysis of movement in the affective behavioral tests
- GraphPad Prism 8 for statistical analysis and data presentation (GraphPad Software Inc., La Jolla, CA)
Procedure
文章信息
版权信息
© 2020 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Montera, M. A. and Westlund, K. N. (2020). Minimally Invasive Oral Surgery Induction of the FRICT-ION Chronic Neuropathic Pain Model. Bio-protocol 10(8): e3591. DOI: 10.21769/BioProtoc.3591.
分类
神经科学 > 神经系统疾病 > 动物模型
神经科学 > 感觉和运动系统 > 动物模型
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link












