- EN - English
- CN - 中文
Lipid Mixing Assay for Murine Myoblast Fusion and Other Slow Cell-cell Fusion Processes
小鼠成肌细胞融合及其他慢细胞融合过程的脂质混合实验
发布: 2020年03月05日第10卷第5期 DOI: 10.21769/BioProtoc.3544 浏览次数: 4931
评审: Khyati Hitesh Shahsujan kumar mondalLiang LiuAnonymous reviewer(s)
Abstract
Lipid mixing (redistribution of lipid probes between fusing membranes) has been widely used to study early stages of relatively fast viral and intracellular fusion processes that take seconds to minutes. Lipid mixing assays are especially important for identification of hemifusion intermediates operationally defined as lipid mixing without content mixing. Due to unsynchronized character and the slow rate of the differentiation processes that prime the cells for cell-cell fusion processes in myogenesis, osteoclastogenesis and placentogenesis, these fusions take days. Application of lipid mixing assays to detect early fusion intermediates in these very slow fusion processes must consider the continuous turnover of plasma membrane components and potential fusion-unrelated exchange of the lipid probes between the membranes. Here we describe the application of lipid mixing assay in our work on myoblast fusion stage in development and regeneration of skeletal muscle cells. Our approach utilizes conventional in vitro model of myogenic differentiation and fusion based on murine C2C12 cells. When we observe the appearance of first multinucleated cells, we lift the cells and label them with either fluorescent lipid DiI as a membrane probe or CellTrackerTM Green as a content probe. Redistribution of the probes between the cells is scored by fluorescence microscopy. Hemifused cells are identified as mononucleated cells labeled with both content- and membrane probes. The interpretation must be supported by a system of negative controls with fusion-incompetent cells to account for and minimize contributions of fusion-unrelated exchange of the lipid probes. This approach with minor modifications has been used for investigating fusion of primary murine myoblasts, osteoclast precursors and fusion mediated by a gamete fusogen HAP2, and likely can be adopted for other slow cell-cell fusion processes.
Keywords: Membrane fusion (膜融合)Background
Fusion of membrane lipid bilayers in cell biological processes as diverse as fusion of intracellular membranes in exocytosis, and fusion of viral and cell membranes in enveloped virus infection, and myoblast fusion in skeletal muscle development apparently involves similar lipid rearrangements (Figure 1) (Brukman et al., 2019). First, a merger of the apposing, contacting monolayers of two fusing bilayers generates a hemifusion connection and allows redistribution of lipid probes between these monolayers. A subsequent merger of the distal monolayers generates a fusion pore and allows redistribution of aqueous probes between fusing membrane compartments.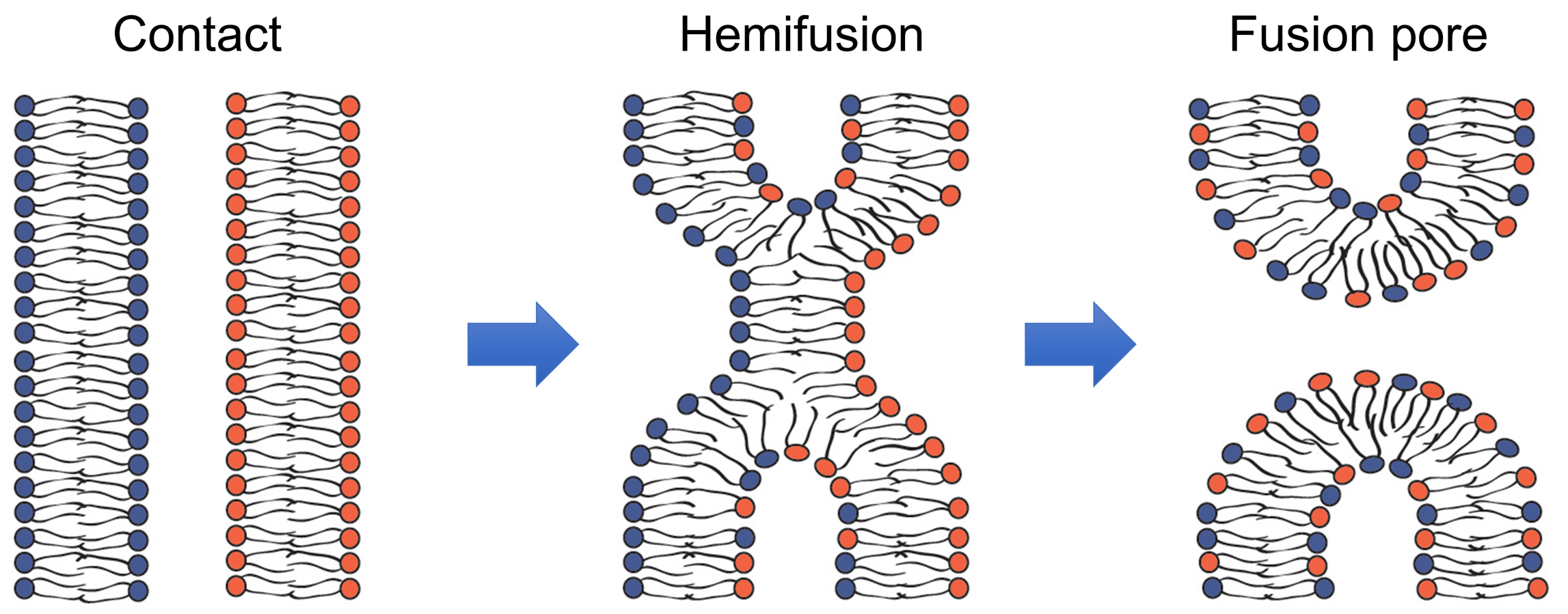
Figure 1. Schematic representation of the lipid rearrangements during hemifusion and fusion pore formation (modified from Figure 1 in Brukman et al., 2019).
Hemifusion can either transition into a fusion pore or represent a dead end of the fusion reaction aborted before fusion pore formation. In the latter case, hemifusion connections can then dissociate yielding two distinct bilayers. In their turn, nascent fusion pores can either close or expand advancing fusion towards its completion with full unification of the membrane compartments. The rates of formation and dissociation of the key fusion intermediates, hemifusion and fusion pores, and the rates of the transition between these intermediates vary between different fusion processes and are determined by the activity of the proteins involved and lipid compositions of the fusing membranes.
In most of the experimental studies, pores large enough to pass content probes in the range from ~1 kDa to ~100 kDa are detected by fluorescence microscopy. Hemifusion, operationally defined as lipid mixing without content mixing, is detected using fluorescence microscopy or spectrofluorometry. Different modifications of lipid mixing assays have been developed in studies on fusion of protein-free lipid bilayers, and fusion mediated by viral fusogens and intracellular fusogens (Brukman et al., 2019). The successful application of the lipid mixing assay in all these systems has been facilitated by a relative rapidness of these fusion processes with characteristic times of lipid mixing varying from seconds to minutes.
Application of lipid mixing assays to developmental cell-cell fusion, the subject of this work, is critically important for clarification of the pathways of the membrane rearrangements but more challenging than for faster viral and intracellular fusion reactions. For instance, formation of multinucleated myotubes, one of the best characterized examples of cell-cell fusion processes (Sampath et al., 2018), is preceded by myogenic differentiation that prepares the cells for fusion and takes days. As a result, by the time fusion is evaluated, fluorescent lipid probes added before fusion to label plasma membranes are already partially internalized. Moreover, labeling of intracellular membrane compartments containing internalized probes may appear brighter than labeling of the plasma membrane because of a higher membrane content and contrast. An additional concern in developing lipid mixing assays for very slow fusion processes is related to the ability of lipid probes to be transferred from membrane to membrane by lipid-exchanging proteins, lipid micelles or extracellular vesicles (reviewed in Merklinger et al., 2016), i.e., by mechanisms that neither involve hemifusion or fusion nor depend on the fusion machinery.
Here we present a protocol used to assay lipid mixing in fusion between C2C12 cells, immortalized mouse myoblasts that proliferate in high-serum medium and differentiate and fuse in low-serum medium. We also discuss applications of the modified versions of this protocol to other slow cell-cell fusion processes.
Materials and Reagents
Materials
- Falcon® 15 ml Polystyrene Centrifuge Tube, Conical Bottom, with Dome Seal Screw Cap (Corning, catalog number: 352095 )
- Tissue culture dishes, 35 x 10 mm REF (Corning, catalog number: 353001 )
- Tissue culture dishes, 60 x 15 mm (Corning, catalog number: 353002 )
Cells
- C2C12 cells (ATCC, catalog number: CRL-1772TM)
- Myomaker-deficient C2C12 cells and Myomerger-deficient C2C12 cells were generated in (Millay et al., 2016; Quinn et al., 2017) and grown on collagen coated substrates
Reagents
- VybrantTM DiI Cell-Labeling Solution (Thermo Fisher Scientific, catalog number: V22885 )
- CellTrackerTM Green CMFDA (5-chloromethylfluorescein diacetate) (Thermo Fisher Scientific catalog number: C7025 )
- Orange CMRA CellTrackerTM (Thermo Fisher Scientific, catalog number: C34551 )
- Trypsin-EDTA 0.05% (Thermo Fisher, catalog number: 25300054 )
- Hoechst 33342 Trihydrochloride, Trihydrate- 10 mg/ml Solution in Water (Thermo Fisher Scientific, catalog number: H3570 )
- Formalin 10% Buffered in Phosphate (Electron microscopy Sciences, catalog number: 15740 )
- Collagen from calf skin (Sigma, catalog number: C8919-20 ml )
- Dimethyl sulfoxide (DMSO, Sigma, catalog number: D2650-100 ml )
- The proliferation medium (PM): DMEM, high glucose, GlutaMAXTM Supplement (Thermo Fisher, catalog number: 10566-016 ) + 10% Fetal Bovine Serum, Penicillin/Streptomycin (Thermo Fisher, catalog number: 10378016 )
- The differentiation medium (DM): DMEM, high glucose, GlutaMAXTM Supplement (Thermo Fisher, catalog number: 10566-016 ) + 5% Horse Fetal Serum, Penicillin/Streptomycin
- Fetal Bovine Serum, FBS (GIBCO Life Technologies, catalog number: 10437-028 )
- Horse Serum, heat inactivated, New Zealand origin (Thermo Fisher, catalog number: 26050088 )
- PBS, Corning® Dulbecco’s Phosphate-Buffered Saline (Life Sciences, catalog number: 21-030-CV ), 1x with calcium and magnesium
Equipment
- Zeiss Axioscope microscope
- Camera (Manufacturer pco-tech inc.; pco.edge 3.1 sCMOS)
- F-LD 32/0.4 Zeiss objective lens
- Single fluorophore bandpass filter for green cell tracker: excitation 472 nm/30 nm, emission 520 nm/35 nm, dichroic 495 nm LP from Semrock
- Single fluorophore bandpass filter for DiI: excitation 545 nm/25 nm, emission 605 nm/70 nm, dichroic 570 LP from Zeiss
Software
- Micro-Manager software (Edelstein et al., 2014)
- The open-source platform, ImageJ (National Institute of Health, Rockville Pike, Bethesda, MD
Procedure
文章信息
版权信息
© 2020 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Leikina, E., Melikov, K., Rabinovich, A. G., Millay, D. P. and Chernomordik, L. V. (2020). Lipid Mixing Assay for Murine Myoblast Fusion and Other Slow Cell-cell Fusion Processes. Bio-protocol 10(5): e3544. DOI: 10.21769/BioProtoc.3544.
分类
发育生物学 > 细胞生长和命运决定 > 肌纤维
生物化学 > 脂质 > 膜脂
细胞生物学 > 细胞成像 > 共聚焦显微镜
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link