- EN - English
- CN - 中文
Rapid Detection of Proliferative Bacteria by Electrical Stimulation
利用电刺激快速检测细菌增殖
发布: 2020年02月05日第10卷第3期 DOI: 10.21769/BioProtoc.3508 浏览次数: 6542
评审: Dong-yeon LeeAkira KarasawaJoe Zhang
Abstract
Detecting live bacteria is an important task for antimicrobial susceptibility testing (AST) in the medical sector and for quality-monitoring in biological industries. Current methods for live-bacteria detection suffer limitations in speed or sensitivity. In a recent paper, we reported that electrical response dynamics in membrane potential enable single-cell rapid detection of live bacteria. The electrical response can be observed within a minute after electrical stimulation. Thus, it has potential in accelerating AST and the monitoring of biological samples. This method also enables experiments for biophysical and microbiological investigations into bacterial electrophysiology. With the hope that more researchers, scientists and engineers will use electrical stimulation for their assays, here we detail each step of the electrical stimulation experiment.
Keywords: Bacterial electrophysiology (细菌电生理学)Background
From fundamental microbiology research to antimicrobial susceptibility testing (AST), quantifying the number of live bacteria is an important task. When it comes to detecting live bacteria, the colony forming unit (CFU) assay, dating from the 19th century, is still the gold standard due to its reliability and sensitivity. However, the CFU assay has a major drawback; it requires 1-3 days of incubation. To overcome this limitation, developments in rapid bacterial detection technology has flourished in recent years (Iqbal et al., 2000; Ahmed et al., 2014). Examples of recently developed technologies are based on impedance spectroscopy, Raman spectroscopy or detection of biomolecules (e.g., ATP, DNA). Nevertheless, overcoming the tradeoff between speed, accuracy and robustness remains a challenge.
Membrane potential indicators are proven useful for distinguishing live and dead bacteria by flow cytometry and fluorescence microscopy (Sträuber and Müller, 2010). However, this method is technically challenging because it requires a careful calibration for bacterial species/strains, media conditions, indicator concentrations, light sources and detectors. In a recent study, we showed that such limitations can be overcome using optical measurements of the electrical response dynamics of membrane potential under stimulation by an external electrical field (Stratford et al., 2019). The responses of proliferative and inhibited cells were distinguishable within a minute; hence, it substantially shortens the time required for a live bacterial assay. Simulations of a phenomenological mathematical model and biophysical understanding suggested that this technology is applicable for different microbial species and antimicrobial treatments. This means that by comparing the electrical dynamics of unperturbed and antimicrobial-treated cells, it may be possible to accelerate AST. An important next step is examining how widely this technology can be used with environmental and pathogenic microbes. This protocol will assist conducting electrical stimulation experiments for AST and live bacteria detection assays with various bacterial species and strains as well as with various antimicrobial treatments.
In addition to its fundamental role in cell proliferation, membrane potential mediates bacterial electrical signaling in biofilms and during the processes of sporulation, mechano-sensation and cell division (Strahl and Hamoen, 2010; Prindle et al., 2015; Bruni et al., 2017; Sirec et al., 2019, Benarroch and Asally, 2020). Membrane potential is also associated with antibiotic resistance (Damper and Epstein, 2010). While these recent studies are beginning to garner attention, membrane potential remains largely overlooked in the field of microbiology. We suspect this oversight is partially due to the technical difficulties associated with experimentally controlling membrane potential. To this end, this protocol will also assist biophysical and microbiological investigations into bacterial electrophysiology.
Materials and Reagents
Notes:
- The following chemicals are interchangeable with appropriate replacements, per the investigator’s discretion.
- All these ingredients can be stored in dry conditions at room temperature.
- Stainless steel mounting wafer
- Copper wires
- Parafilm
- 22 x 22 mm glass slip
- Microscope Coverslips
- Bacterial samples
- Titanium-Gold Alloy
- DifcoTM Agar, Granulated (VWR, BD Biosciences, catalog number: 90000-786)
- L-Glutamic acid monosodium hydrate (Sigma-Aldrich, catalog number: G1626)
- Glycerol 99% purity (Fisher Chemical, catalog number: G/0650/17)
- 3-(N-morpholino)propanesulfonic acid (MOPS) (Sigma-Aldrich, catalog number: M9381)
- Potassium Phosphate dibasic Trihydrate, K2HPO4·7H2O (Sigma-Aldrich, catalog number: 431478)
- Potassium Dihydrogen Orthophosphate, KH2PO4 (Fisher Chemical, catalog number: 7778-77-0)
- D-Glucose (Sigma-Aldrich, catalog number: D9434)
- Ammonium Chloride, NH4Cl (Fisher Chemical, catalog number: 12125-02-9)
- Calcium Chloride, CaCl2·2H2O (Fisher Chemical, catalog number: 10035-04-8)
- Magnesium Chloride, MgCl2 (Fisher Chemical, catalog number: 7791-18-6)
- Iron (III) Chloride, FeCl3·6H2O (Fisher Chemical, catalog number: 10025-77-1)
- Manganese Chloride, MnCl2 (Sigma-Aldrich, catalog number: 244589)
- Zinc Chloride, ZnCl2 (Fisher Chemical, catalog number: 7646-85-7)
- Thiamine Hydrochloride (Sigma-Aldrich, catalog number: T4625)
- Thioflavin T (Sigma Aldrich, catalog number: T3516)
- NaOH
- LB Broth (Fisher Scientific, catalog number: BP1426-2)
- 0.5 M potassium phosphate buffer (pH 7) (see Recipes)
- MOPS buffer (see Recipes)
- Modified MSgg (see Recipes)
Equipment
Note: The list of the specific equipment used in Stratford et al., 2019 can be found in Tables 1-2.
Essential Equipment
- Inverted Fluorescence Microscope
- Microscope Incubation Chamber
- Temperature Controller
- Fluorescent Light Source
- Arbitrary Function Generator
- Bespoke electrode dishes (The bill of materials is in Table 3)
- Relay Circuit (The diagram is in Figure 4)
- Electron Beam Vapor Deposition System or equivalent alloy deposition method
Supplementary Equipment
- pH meter or testing strips
- Weighing scales
- Shaking incubator
- Adjustable volume pipettes ranging from 0.1 μl to 1,000 μl
- Measuring cylinder or serological pipettes ranging from 5-100 ml
- Scalpel
- Tweezers
- Bunsen Burner
Table 1. List of equipment for Example Essential Equipment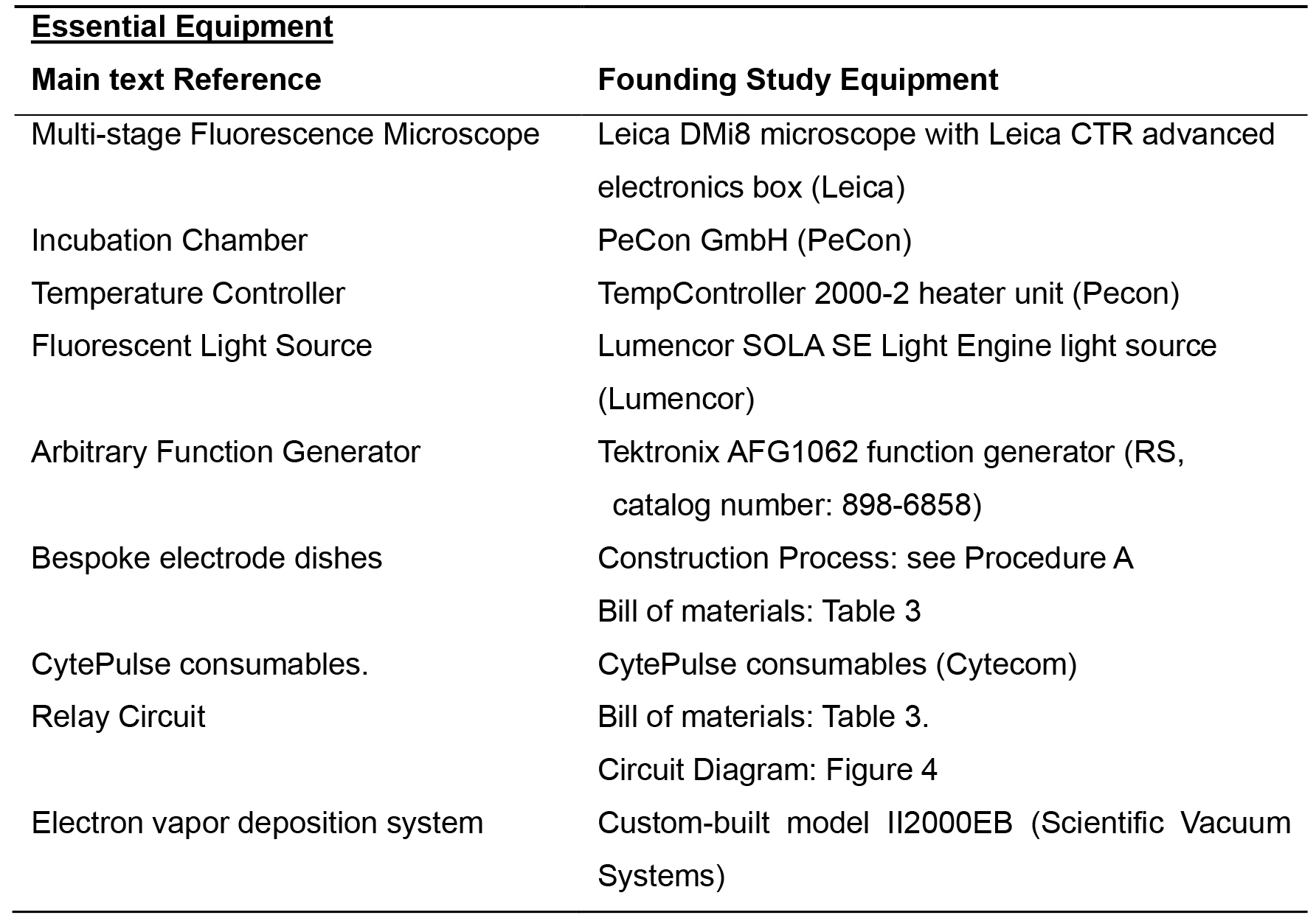
Table 2. List of equipment for Example Supplementary Equipment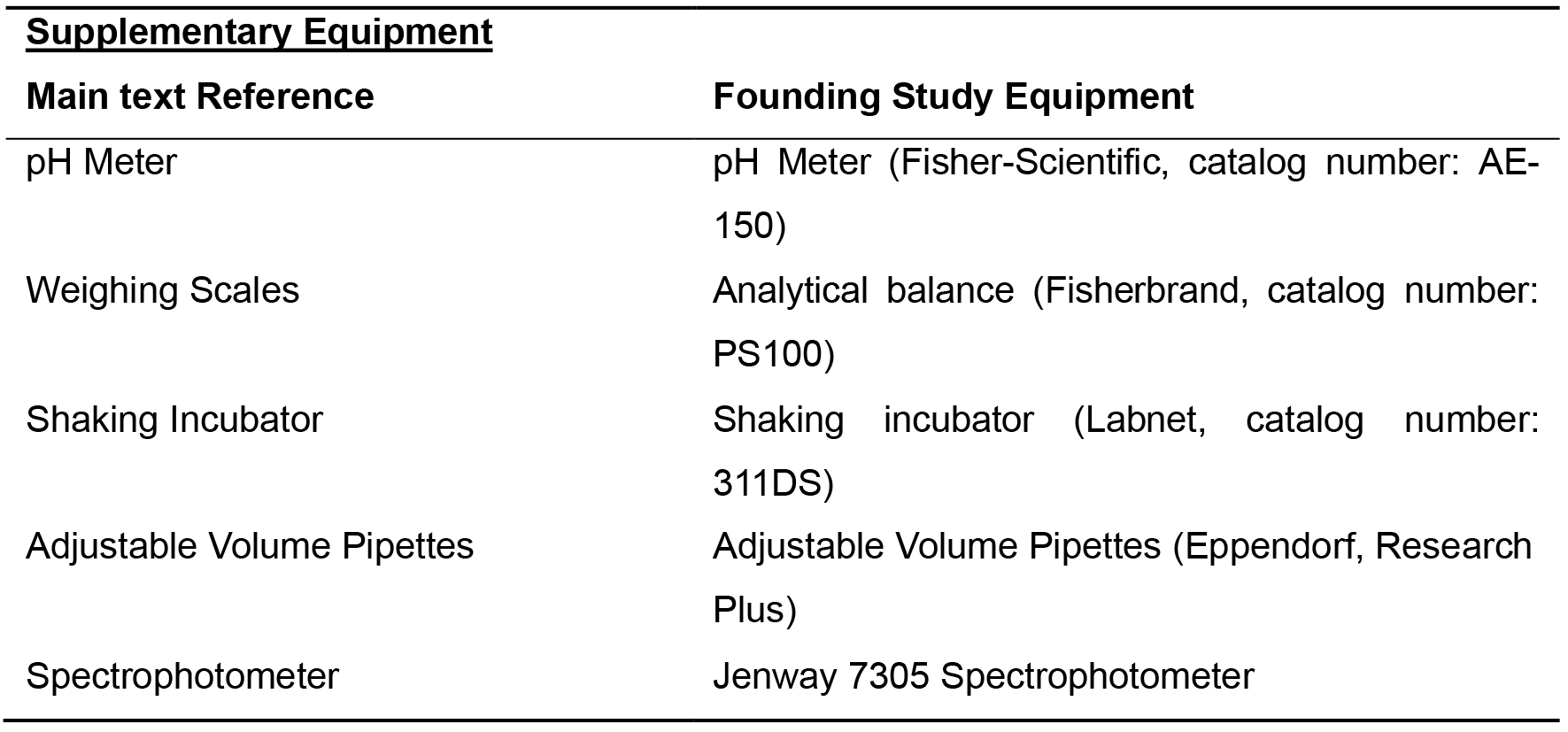
Table 3. Bespoke electrode dish bill of materials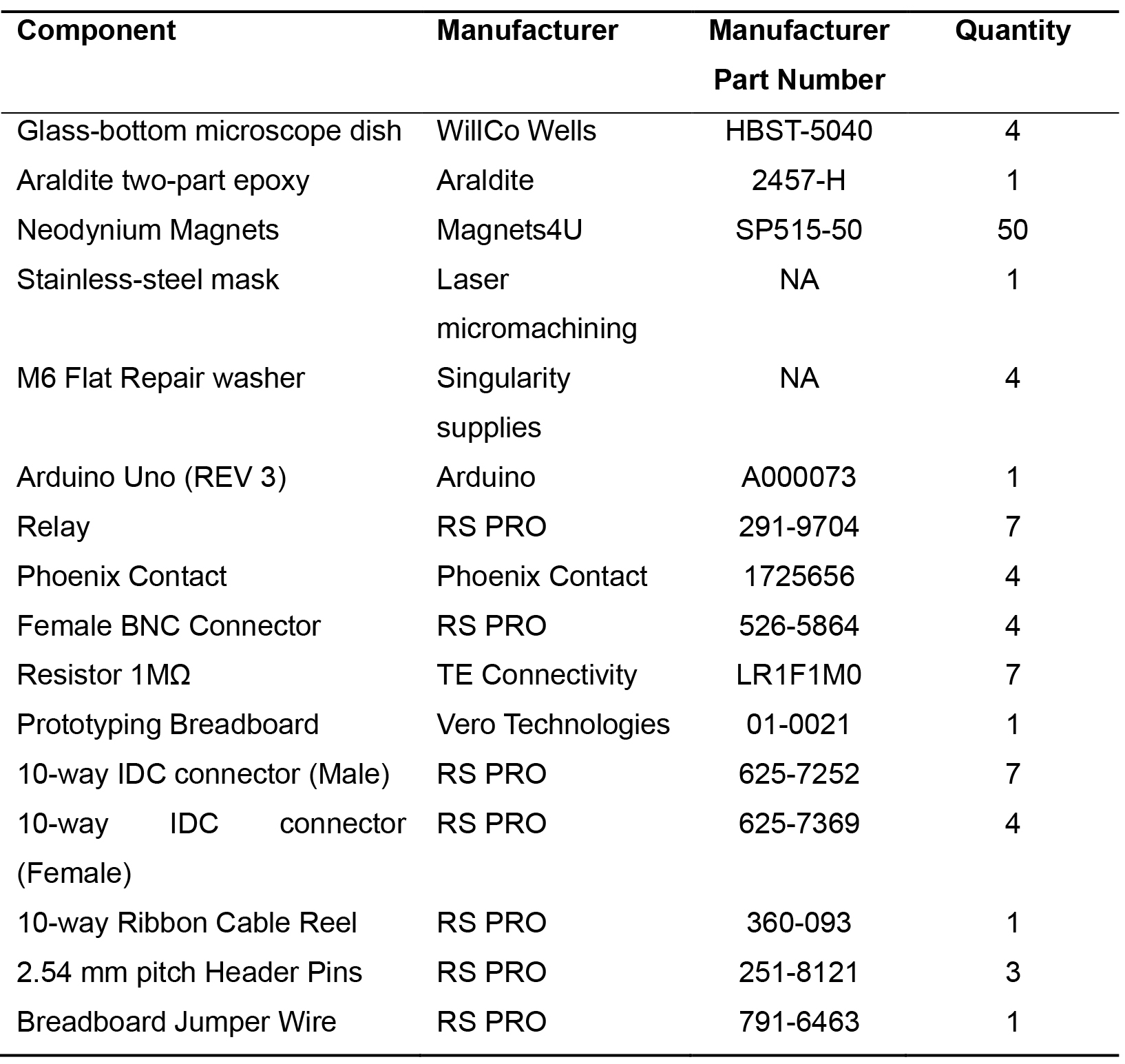
Software
- Microscope Control Software (e.g., MetaMorph, micro-manager [micro-manager.org/])
- Arduino IDE (arduino.cc) (not necessary with Cytepulse method)
- Image processing software (e.g., Fiji/ImageJ (fiji.sc) (Schindelin et al., 2012))
- Data processing software (e.g., Matlab, Anaconda Python/R Distribution [anaconda.com]).
Procedure
文章信息
版权信息
© 2020 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
LA Edwards, C., Malyshev, D., Stratford, J. P. and Asally, M. (2020). Rapid Detection of Proliferative Bacteria by Electrical Stimulation. Bio-protocol 10(3): e3508. DOI: 10.21769/BioProtoc.3508.
分类
微生物学 > 抗微生物试验 > 抗细菌试验
微生物学 > 微生物细胞生物学 > 细胞成像
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link