- EN - English
- CN - 中文
Identification of Heteroreceptors Complexes and Signal Transduction Events Using Bioluminescence Resonance Energy Transfer (BRET)
利用生物发光共振能量转移进行异受体复合物和信号转导的鉴定
(*contributed equally to this work) 发布: 2019年10月05日第9卷第19期 DOI: 10.21769/BioProtoc.3385 浏览次数: 4992
评审: Edgar Soria-GomezJoana Alexandra Costa ReisMaria Nguyen

相关实验方案

基于蛋白筛选策略从合成文库中分离抗原特异性纳米抗体:结合 MACS 的酵母展示筛选与 FLI-TRAP 方法
Apisitt Thaiprayoon [...] Dujduan Waraho-Zhmayev
2026年01月20日 473 阅读
Abstract
Detecting protein-protein interactions by co-immunoprecipitation provided a major advancement in the immunology research field. In the G-protein-coupled receptors (GPCRs) research field, colocalization and co-immunoprecipitation were used to detect interactions, but doubts arose due to specificity of the antibodies (monoclonal in the case of receptors related to immunology and polyclonal in the case of GPCRs) and due to the possibility of false positive due to the potential occurrence of bridging proteins. Accordingly, new methodological approaches were needed, and energy transfer techniques have been instrumental to detect direct protein-protein, protein-receptor or receptor-receptor interactions. Of the two most relevant methods (Förster, or fluorescence resonance energy transfer: FRET and Bioluminescence energy transfer: BRET), the protocol for BRET is here presented. BRET has been instrumental to detect direct interactions between GPCRs and has contributed to demonstrate that GPCR dimers/oligomer functionality is different from that exerted by individual receptors. Advantages outweigh those of FRET as no fluorescence source is needed. Interestingly, BRET is not only useful to validate interactions detected by other means or hypothesized in the basis of indirect evidence, but to measure signal transduction events. In fact, BRET may, for instance, be used to assess β-arrestin recruitment to activated GPCRs.
Keywords: G-protein-coupled receptors (G蛋白偶联受体)Background
Fluorimeters have been in research laboratories for decades and still are. Also, cell biology took advantage of fluorescent detection of molecules using antibodies conjugated to small fluorescent molecules. A next stage was reached upon the discovery and further development of big fluorescent molecules. A fluorescent protein may be fused to a protein of interest to detect it even in living cells; now it is even possible to follow proteins in tissues of transgenic animals engineered to express the fluorescent fusion protein. Here are now available a variety of proteins with different fluorometric properties, i.e., with different wavelengths of absorption and/or emission. The Nobel Prize in Chemistry in 2008 was awarded to O. Shimomura, M. Chalfie and R. Y. Tsien: “for the discovery and development of the green fluorescent protein, GFP”. The press note indicated: “By using DNA technology, researchers can now connect GFP to other interesting, but otherwise invisible, proteins. This glowing marker allows them to watch the movements, positions and interactions of the tagged proteins” (https://www.nobelprize.org/prizes/chemistry/2008/press-release/).
Energy transfer protocols are now instrumental to detect molecular interactions, which were previously suspected by colocalization and “confirmed” by co-immunoprecipitation, i.e. by bringing down one protein using a monoclonal antibody against the “interacting” partner (Burger et al., 1986; Springer et al., 1986; Dianzani et al., 1999; Serrador et al., 1999). Unfortunately, it was not (still is not) easy to develop monoclonal antibodies against G-protein-coupled receptors (GPCRs). The first one produced to detect a GPCR, the adenosine A2A receptor, is probably still the best one (Rosin et al., 1998). But the majority of “good” anti-GPCR antibodies are polyclonal and the first ones appearing in the literature for class A GPCRs recognized adenosine receptors (Ciruela et al., 1995; Jimenez et al., 2008). Many of the antibodies against GPCRs are polyclonal and were used for coimmunoprecipitation (Ginés et al., 2000). Critiques arrived soon from two points of view: doubts on the specificity of polyclonal antibodies and the possibility that in detergent-treated cells a (third) bridging protein could lead to artifacts.
Energy transfer between proteins can only occur if the donor and the acceptor are at a distance of < 10 nm. The first option is using two proteins of interest each fused to a different fluorescence protein. Such approach is known as Förster resonance energy transfer: FRET (Förster, 1948; Perkins et al., 1984). In parallel Bioluminescence resonance energy transfer: BRET, was developed in the basis of a bioluminescent donor and a fluorescent acceptor (Xu et al., 1999). This is the technique whose protocol will be detailed here. Conceptually, the technique requires one protein fused to an enzyme (Renilla reniformis luciferase; a protein of circa 36 kDa) that degrades a substrate and emits light that in turns excite another protein fused, for instance, to the yellow fluorescent protein (YFP) (Figure 1). Two possible BRETs exist but we will detail BRET1, as the protocol would be quite similar with BRET2 (the main two differences are the substrate of Rluc and the fluorescent acceptor). BRET can also be used for signal transduction measurements. Probably the best example in the field of GPCRs is the measurement of β-arrestin binding to activated GPCRs. In that case the β-arrestin is fused to Rluc and acts as donor of the fluorescent protein fused to the GPCR of interest. BRET is repeated at different times to get time-response curves of β-arrestin recruitment or at a given time but with different doses of the GPCR activator to get dose-response curves. Reviews on the BRET technique (including the so-called nano-BRET) and on different possibilities that it offers in different fields and/or to answer scientific questions can be elsewhere found (Pfleger and Eidne, 2005; Breton et al., 2010; Schann et al., 2013; Beautrait et al., 2014; Sun et al., 2016; Dale et al., 2019).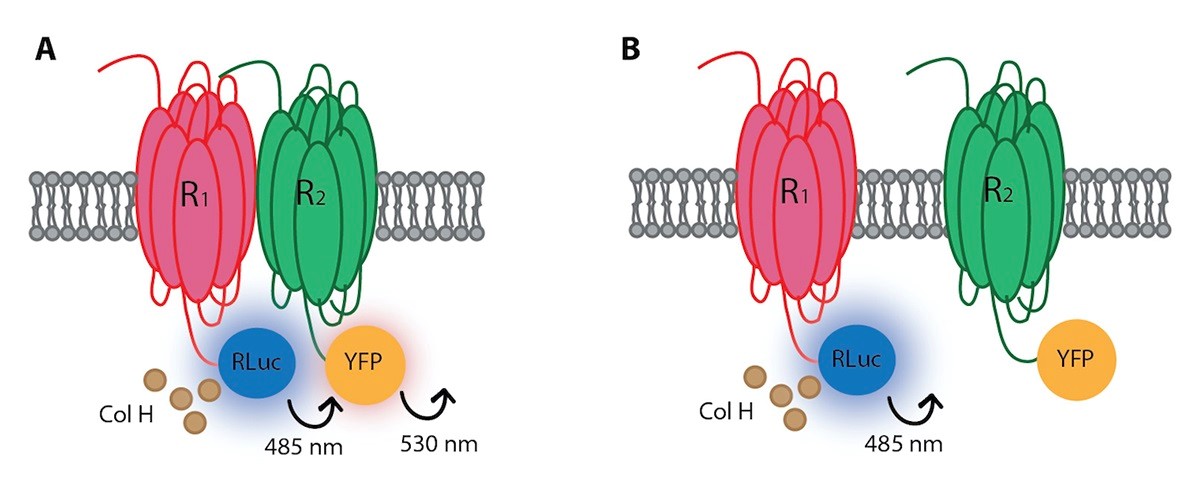
Figure 1. Cartoon representation of the BRET assay between a fusion protein constituted by R1 and Rluc and another fusion protein constituted by R2 and YFP. A. When both receptors (R1 and R2) are in close proximity (distance ≤ 100 A) there is energy transfer from Rluc-derived bioluminescence to YFP. B. When receptors are at a higher distance, no energy transfer can be detected. Rluc: Renilla luciferase. Col H: coelenterazine H, a substrate of Rluc. YFP: Yellow fluorescent protein. R1: cell surface receptor number 1. R1: cell surface receptor number 2.
Materials and Reagents
- Sterilization 0.22 µm pore size filter, 33 mm diameter (Millipore, catalog number: SLGP033RS)
- Sterile 6-well plates for cell culture (Cultek, catalog number: 153506)
- Transparent 96-well microplates (Sudelab, catalog number: 900011)
- 96-well black micro plates with a transparent bottom (Biogen, catalog number: PO-215003)
- 96-well white microplates with white bottom (Biogen, catalog number: PO-204003)
- Sterile 15 ml centrifuge falcon tubes (Cultek, catalog number: 45352096)
- Sterile 2 ml Eppendorf tubes
- Human embryonic kidney (HEK-293T) cells [293T (ATCC®, catalog number: CRL-3216TM)], store aliquots in liquid nitrogen
- Plasmids for cell transfection
Note: One containing the code for one protein fused to Rluc and another containing the code for the second protein fused to enhanced yellow fluorescent protein (EYFP). Plasmids including the sequences for Rluc or YFP are available from ADDGENE, store at -20 °C. - L-glutamine (Gibco, catalog number: 25030-024), store at -20 °C
- Penicillin/streptomycin (Gibco, catalog number: 15140-122), store at -20 °C
- Sodium chloride (NaCl) (PanReacApplichem, catalog number: 141659.1211), store solid at room temperature, or 150 mM aliquots at 4 °C
- NaCl (Panreac, catalog number: 131659.1211), store at room temperature
- Ethanol
- CaCl2·2H2O (Panreac, catalog number: 131232.1211), store at room temperature
- KCl (Panreac, catalog number: 131494.1211), store at room temperature
- KH2PO4 (Panreac, catalog number: 141509.1211), store at room temperature
- MgCl2, anhydrous (Sigma-Aldrich, catalog number: M8266), store at room temperature
- MgSO4·7H2O (Panreac, catalog number: 141404.1211), store at room temperature
- Na2HPO4·12H2O (Panreac, catalog number: 141678.1211), store at room temperature
- HEPES (Panreac, catalog number: A3724,0500), store at room temperature
- Glucose (PanReac, catalog number: A1422,1000), store at room temperature
- Bradford reagent (BioRad, catalog number: 500-0006), store at 4 °C
- Bovine serum albumin (Sigma-Aldrich, catalog number: A6003-25g) store either solid at 4 °C, or in solution at -20 °C
- Coelenterazine H (Attendbio, catalog number: E-102181), store at -20 °C, protected from light
- Dulbecco’s modified Eagle’s medium (DMEM) (Gibco, catalog number: 21969-035), store at 4 °C
- MEM Non-Essential Amino Acids Solution (Gibco, catalog number: 11140-035), store at 4 °C
- Fetal Bovine Serum (FBS) (Gibco, catalog number: 10270106), store at -20 °C
- Polyethylenimine (PEI) (Sigma-Aldrich, catalog number: 408727), store either solid at room temperature, or in 40 µM aliquots at -20 °C
- Sterile 40 µM PEI (see Recipes)
- Sterile 150 mM NaCl (see Recipes)
- Serial BSA dilutions (0-1 mg/ml) (see Recipes)
- Supplemented DMEM (see Recipes)
- HBSS (see Recipes)
- Coelenterazine H solution (see Recipes)
Equipment
- Water bath (Kotterman, model: D-3162)
- Centrifuge (Eppendorf, model: 5084R)
- Centrifuge (Eppendorf, model: 5415R)
- Cell incubator (ThermoFisher, model: 3308)
- Laminar flow hood (Faster, model: Bio60)
- Spectrophotometer (ThermoLabsystems, multiskan Ascent; 354)
- Fluostar Optima Fluorimeter (BMG Labtech, FLUOstar OPTIMA) equipped with a high-energy xenon flash lamp, a 10 nm bandwidth excitation filter at 485 nm and a 530 nm emission filter
- Mithras LB 940 plate reader (Berthold Technologies) equipped with 485 nm and 530 nm filters
Software
- GraphPad Prism software (GraphPad, https://www.graphpad.com/scientific-software/prism/)
- Microsoft Excel (Microsoft, https://products.office.com/es/excel)
- Multiskan software, provided by manufacturer of the Plate reader for measuring protein concentration
- OPTIMA software, provided by manufacturer of the fluorescence plate reader
- Mikrowin 2000 software, provided by manufacturer of the BRET reader
Procedure
文章信息
版权信息
© 2019 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Reyes-Resina, I., Jiménez, J., Navarro, G. and Franco, R. (2019). Identification of Heteroreceptors Complexes and Signal Transduction Events Using Bioluminescence Resonance Energy Transfer (BRET). Bio-protocol 9(19): e3385. DOI: 10.21769/BioProtoc.3385.
分类
神经科学 > 基础技术 > 受体-受体互作
分子生物学 > 蛋白质 > 蛋白质-蛋白质相互作用
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link











