- EN - English
- CN - 中文
Isolation of Pure Mitochondria from Rat Kidneys and Western Blot of Mitochondrial Respiratory Chain Complexes
大鼠肾脏单纯线粒体的分离和线粒体呼吸链复合物蛋白质免疫印迹
发布: 2019年10月05日第9卷第19期 DOI: 10.21769/BioProtoc.3379 浏览次数: 11332
评审: Xiaoyi ZhengDhanya K NambiarMei Po Chan
Abstract
Cardiac, neuronal and renal tubular epithelial cells are the most metabolically active cells in the body. Their fate depends largely on their mitochondria as the primary energy generating system which participates in the control of apoptosis, cell cycle and metabolism. Thus, mitochondrial dysfunction is a hallmark of many chronic diseases including diabetic nephropathy. A drop in mitochondrial bioenergetics efficiency is often associated with altered expression of respiratory chain complexes. Moreover, recent studies demonstrate that cellular proteins can shuttle to mitochondria and modify their function directly. Here we illustrate two mitochondria isolation protocols; one is recommended if the purity of the mitochondrial fraction is a priority such as if the mitochondrial localization of a protein has to be validated, the other if a high yield of intact functional mitochondria is required for functional studies and quantitative Western blotting. Next, we provide a detailed protocol for Western blotting of isolated mitochondria and renal cortex either to prove the purity of isolated fractions or to quantify complexes of the mitochondrial respiratory chain. We used this approach to identify classically cell membrane bound angiotensin II receptors in mitochondria and to study the effect of these receptors on mitochondrial function in early stages of diabetic nephropathy.
Keywords: Mitochondria (线粒体)Background
Mitochondria are the primary energy generating system in metabolically active cells with a high energy demand such as renal proximal tubular epithelial cells (PTEC), cardiac muscle and neuronal cells. Additionally, they participate in the regulation of calcium signaling, proliferation, cell death and metabolism (Antico Arciuch et al., 2012). Thus, it is not surprising that diabetic nephropathy (DN) correlates more closely to defective mitochondria and increased oxidative stress in the kidney than to hyperglycemia. Increased reactive oxygen species (ROS) generated by defective mitochondria may initiate a vicious cycle, aggravating oxidative stress and mitochondrial dysfunction (Arora and Singn, 2014; Higgins and Coughlan, 2014). Mitochondrial ATP is produced in the respiratory chain (ETC), an assembly of more than 20 discrete electron carriers that are mainly grouped into five multi-polypeptide complexes and localized to the inner mitochondrial membrane. Increased oxygen consumption for ATP production may contribute to ischemia. Increased ROS may alter a number of functional cellular processes. A major regulator of DN is angiotensin II (AngII) which primarily acts via its type 1 receptor (AT1R). A second AngII receptor type 2 (AT2R) often counteracts the AT1R mediated effects (Peters et al., 2012). We have previously shown that mitochondrial dysfunction already occurred in the very early stages of DN and was associated with an altered expression of the ETC complexes. Further we demonstrated that the AT2R, classically located at the cell membrane as a G-protein coupled receptor, can translocate to the mitochondria and protect against the diabetes induced drop in mitochondrial bioenergetics efficiency, the rise in mitochondrial superoxide production, metabolic reprogramming and increased PTEC proliferation. These effects were associated with altered expression of ETC complexes (Micakovic et al., 2018). Such translocation of cellular proteins to the mitochondria was recently reported for a number of proteins including renin (Peters et al., 1996; Wanka et al., 2009), ACE2 (Wilson et al., 2016), vitamin D receptor (Silvagno et al., 2013) and polycystin 1 (Lin et al., 2018). In order to prove their presence in mitochondria and to dissect their mitochondrial function from indirect effects, studies on isolated mitochondria are required. Isolation of intact, functional and pure mitochondria is crucial for in vitro approaches. Furthermore, we performed Western blotting to quantify the ETC complexes and to evaluate the mitochondrial fraction for possible contaminations with other cellular components like the endoplasmatic reticulum, cytosol and cell membrane. Mitochondrial isolation procedures still vary among research groups and inconsistent or contradictory results often found in the literature may be due to differences in the quality of mitochondria as a result of different isolation procedures (Gross et al., 2011).
In this paper, we illustrate two protocols with highly reproducible outcomes for the isolation of pure and structurally intact mitochondria from kidney cortex of experimental rats: 1. isolation using the QproteomeTM Mitochondria Isolation Kit and 2. isolation using the serial differential centrifugation method. Isolation of mitochondria using QproteomeTM Mitochondria Isolation Kit provides highly pure mitochondrial fraction, free of contamination by other cellular compartments. The main limitation of this method is that it uses only 60 mg of tissue and therefore results in a low mitochondrial yield and is very expensive. This method is performed according to the manufacturer’s protocol, with slight changes. During the isolation procedure, different subcellular fractions can be collected (cytosol, nuclei/cell debris, mitochondria, and plasma membrane/endoplasmatic reticulum (ER). This isolation method is recommended if the purity of the mitochondrial fraction is a priority, such as if the mitochondrial localization of a protein has to be validated or composition of the mitochondrial proteome has to be determined by mass spectroscopy (Figure 1A). Isolation of mitochondria using the differential centrifugation method provides a much higher yield of functionally active mitochondria. The mitochondrial fraction obtained has a slight reduction in purity compared to the QproteomeTM Mitochondria Isolation Kit, but still has very low levels of contamination (> 90% of purity). This isolation method can be used for mitochondrial functional studies which require a large quantity of structurally intact mitochondria. The homogenization procedure is a crucial step that can significantly impact the quality of resulting mitochondria. However, the isolation methods that result in high fractional yield typically require harsh homogenization that often damages the organelles. In contrast to other published mitochondria isolation protocols which harvested the 3,000-10,000 x g fraction (Graham, 2001), we used a very gentle homogenization in Dounce glass homogenizer with a manually driven glass pestle. We then harvested the 500-1,000 g fraction rich in pure mitochondria with preserved integrity after differential centrifugation at 100, 300, 500 and 1,000 x g (Peters et al., 1996). This approach minimizes both mitochondria damage and contamination with the microsomes/plasma membranes and nuclei (Figure 1B).
For validation of purity and structural integrity of isolated mitochondria, we describe a detailed protocol for Western blot performed on subcellular fractions or proteins isolated from the whole kidney cortex. To demonstrate the purity of the isolated mitochondrial fraction we used antibodies targeting a specific organelle marker: the presence of endoplasmic reticulum is detected by Anti-GRP78; cytosol by Anti-GAPDH; and nucleus by Anti-Histone H3. The presence of the endoplasmic reticulum is also detected by using Anti-KDEL monoclonal antibody. Anti-COX IV antibody is used as an effective loading control for mitochondria (Figures 1A and 1B). COX IV is polypeptide chains of cytochrome c oxidase, the terminal oxidase in mitochondrial electron transport, and it is generally expressed at a consistently high level. Mitochondrial integrity is an important consideration when assessing the function of isolated mitochondria since damaged mitochondrial membranes can become rate limiting factor for oxygen consumption and ATP synthesis. We detected representative subunits of each electron transport chain complex (ETC) (CI:NDUFB8, CII:SDHB, CIII:UQCRC2, CIV:MTC01, CV:ATPVA) in mitochondrial fraction using an OXPHOS cocktail of monoclonal antibodies. Anti VDAC-1/Porin monoclonal antibody is used to detect the mitochondrial outer membrane.
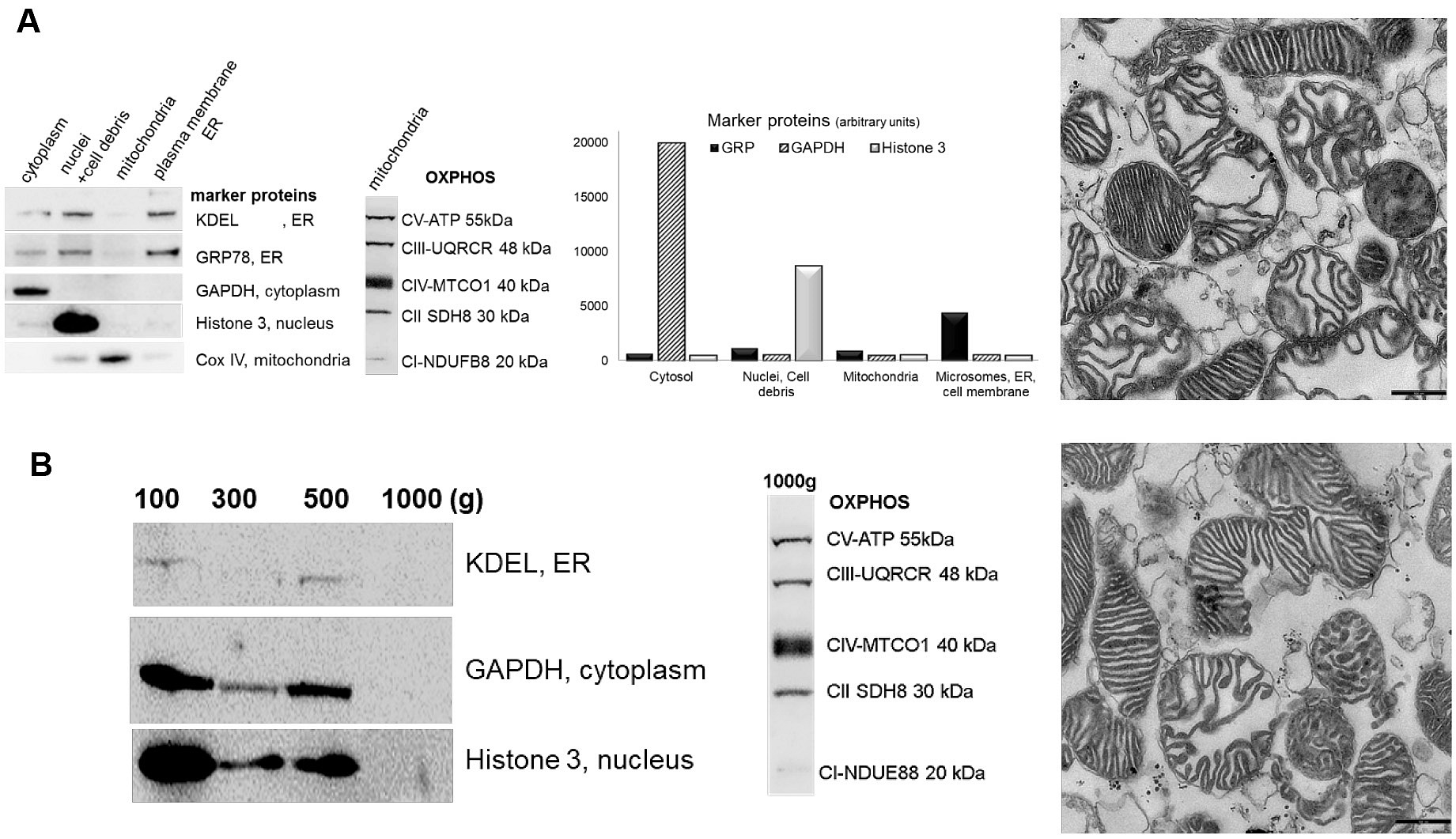
Figure 1. Western blot (left) and electron microscopy (right) demonstrating purity of mitochondrial fractions. A. QproteomeTM Mitochondria Isolation Kit. B. Differential centrifugation. Western blot of subcellular preparations probed with antibodies specific for organelle/cell compartment specific marker proteins: cytosol (GAPDH), nucleus (Histone 3), mitochondria (COXIV-ab16056, abcam) and microsome/ER/PM (KDEL-ab1223, abcam, GRP78) (GAPDH, GRP78, Histone 3 ab139415, abcam) fractions (left). OXPHOS antibody cocktail-ab110413, abcam Figure 2).
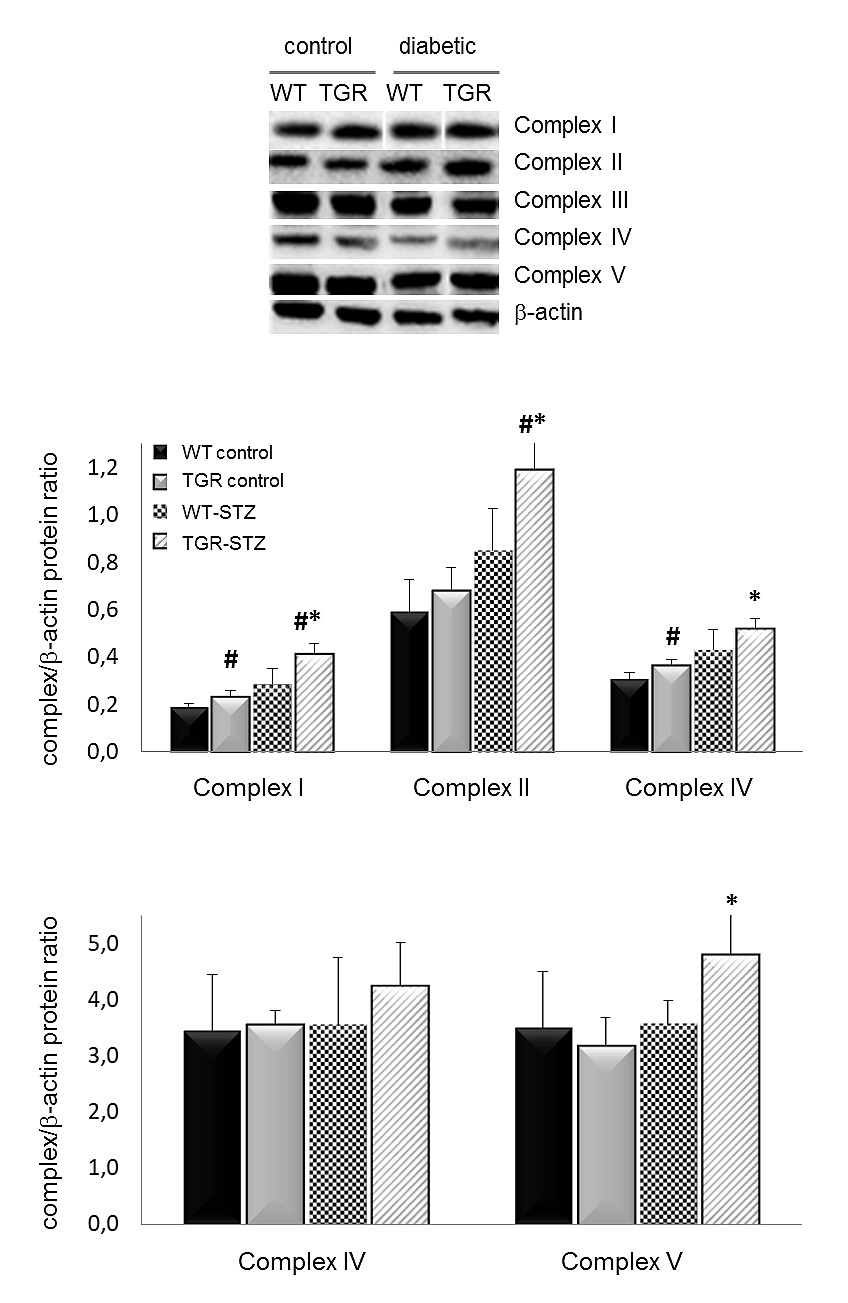
Figure 2. Western Blot of mitochondrial respiratory chain complexes. All complexes of the respiratory chain, except complex III, are increased in diabetic TGRs relative to their nondiabetic controls and diabetic WT-rats. The antibodies are against a subunit of each complex (CI:NDUFB8, CII:SDHB, CIII:UQCRC2, CIV:MTC01, CV:ATPVA) that is labile when its complex is not assembled. Results are expressed as means ± SD. *P ≤ 0.05 vs. control; #P ≤ 0.0 vs. wildtype, 3-4 rats/group. WT–wildtype rats, TGR–transgenic rats. STZ streptozotocin-diabetic.
Materials and Reagents
- Materials
- Pipettes tips
- Glass bottle with in- and outtake socket
- Bulldog-clamp
- Plastic tray
- Adhesive tape
- Scissors
- Disposable Scalpel (Swann-Morton, Disposable Scalpel, catalog number: 0086)
- Razor blade (Häberle Labortechnik GmbH, Germany, catalog number: 6800353)
- Butterfly-19 gauge (OD-Ø 1.1 mm; ID-Ø 0.8 mm)
- Bulldog Clamp, Johns Hopkins bulldog clamp, straight, 3,8 cm (Fisher scientific, catalog number: 12-460-556)
- Einmal-Küvetten aus PS (neoLab Migge GmbH, ecoLab, catalog number: E-1642)
- Eppendorf Safe-Lock Tubes 2 ml, 1,5 ml (Eppendorf AG, Eppendorf Tubes®)
- Filter Paper (Merck KGaA, Whatman® cellulose chromatography papers, catalog number: WHA3030917)
- Homogeniser POTTER-ELVEHJEM with Glass pestle, 23 (17 ml) (Otto E. Kobe KG, catalog number: 9.651 677)
- Wheaton Mikro-Gewebe-Handhomogenisator Dounce 1 ml, with loose and tight glass pestle (NeoLab, catalog number: 9-0972)
- Homogeniser with glass pestle, 3 ml (NeoLab, catalog number: 9-0936)
- Injection needle (Becton, Dickinson, model: BD Microlance 27G REF300635)
- NuncTM 15 ml and 50 ml Conical Sterile Polypropylene Centrifuge Tubes (Thermo Fisher Scientific, NuncTM Centrifuge Tubes)
- NuPAGETM 4-12% Bis-Tris Protein Gels (Thermo Fisher Scientific, InvitrogenTM, catalog number: NP0321BOX)
- Petri dish 100 mm (Thermo Fisher Scientific, Nunc, catalog number: 4031)
- Petri dish 35 mm (Santa Cruz Biotechnology, catalog number: sc200284)
- Pipette tips (STARLAB INTERNATIONAL GmbH, TipOne®)
- PVDF-Membrane (Merck KGaA, Immobilon®, catalog number: IPVH00010)
- Razor blade (Häberle Labortechnik GmbH, Germany, catalog number: 6800353)
- Tuberkulin syringe Luer Lok, 1 ml Omnifix-F, oneway sterile (Fa. B. Brown, catalog number: 9161406V)
- XCell IITM Blot Module (Thermo Fisher Scientific, InvitrogenTM, catalog number: EI9051)
- XCell SureLock® Mini-Cell gel running tank (Thermo Fisher Scientific, InvitrogenTM, catalog number: EI0001)
- Reagents
- Antibodies
- Anti-COX IV Antibody, rabbit polyclonal to COX IV (Abcam, catalog number: ab16056), storage at -80 °C, dilution 1:1,000
- Anti-KDEL Antibody, mouse monoclonal [10C3] to KDEL (Abcam, catalog number: ab12223), storage at -80 °C, dilution 1:1,000
- Anti-VDAC1/Porin Antibody, mouse monoclonal [20B12AF2] to VDAC1/Porin (Abcam, catalog number: ab14734), storage at 4 °C, dilution 1:800
- Anti-β-Actin Antibody, mouse monoclonal to β-Actin (Sigma-Aldrich, catalog number: A2228), storage at -20 °C, dilution 1:2,000
- Endoplasmic Reticulum Fraction Western Blot Cocktail (GAPDH, GRP78, Histone 3) (Abcam, catalog number: ab139415), storage at -20 °C, dilution 1:500
Note: The cocktail already contains the HRP Conjugated Secondary Antibody Cocktail. - Goat anti-Mouse IgG (H+L) Secondary Antibody, HRP (Thermo Fisher Scientific, catalog number: 32430), storage at 4 °C, dilution 1:2,000
- Goat anti-Rabbit IgG Secondary Antibody, HRP (Santa Cruz Biotechnology, catalog number: sc-2004), storage at 4 °C, dilution 1:2,000
- Total OXPHOS Rodent WB Antibody Cocktail (Abcam, catalog number: ab110413), storage at 4 °C, dilution 1:750
Note: The cocktail contains 5 mouse m-antibodies, one each against a subunit of each complex (CI:NDUFB8, CII:SDHB, CIII:UQCRC2, CIV:MTC01, CV:ATPVA), Bio-Rad Protein Assay Dye Reagent Concentrate (Bio-Rad Laboratories, catalog number: 500-0006), storage at 4 °C.
- Bovine Serum Albumine (Sigma-Aldrich, catalog number: A3311-50G)
- Mannitol, M = 182.17 g/mol (Sigma-Aldrich, catalog number: M9546)
- EDTA·2H2O, M = 372.24 g/mol (AppliChem, catalog number: A3553)
- EGTA, M = 380.4 g/mol (Sigma-Aldrich, catalog number: E3889)
- Heparin-sodium (Sigma-Aldrich, catalog number: H0200000)
- IGEPAL® CA-630 (Sigma-Aldrich, catalog number: 9036195)
- Magnesium Chloride, 6-Hydrate, Crystal, U.S.P. (MgCl2·6H2O), M = 203.3g/mol (J.T. Baker, catalog number: 7791-18-6)
- Morpholinopropane Sulfonic Acid (MOPS), M = 209.3 g/mol (Serva, catalog number: 29836)
- NuPAGETM LDS Sample Buffer (4x) (Thermo Fisher Scientific, InvitrogenTM, catalog number: NP0007), storage at 4 °C
- NuPAGETM MES SDS Running Buffer (20x) (Thermo Fisher Scientific, InvitrogenTM, catalog number: NP0002), storage at 4 °C
- NuPAGETM Sample Reducing Agent (10x) (Thermo Fisher Scientific, InvitrogenTM, catalog number: NP0004), storage at 4 °C
- NuPAGETM Transfer Buffer (20x) (Thermo Fisher Scientific, InvitrogenTM, catalog number: NP0006)
- Phenylmethanesulfonyl fluoride (PMSF), M = 174.19 g/mol (Gibco BRL, catalog number: 15521-016)
- Potassium Phosphate Dibasic Trihydrate (K2HPO4·3H2O), M = 228.22 g/mol (Sigma-Aldrich, catalog number: P966)
- cOmpleteTM Protease Inhibitor Cocktail (Sigma-Aldrich, catalog number: 11836145001), storage at 4 °C
- Qproteome Mitochondria Isolation Kit (Qiagen, catalog number: 37612)
- SeeBlue Plus2 Prestained Standard (Thermo Fisher Scientific, InvitrogenTM, catalog number: LC5925), storage at 4 °C
- Sodium Chloride (Sigma-Aldrich, catalog number: S3014)
- Sodium Deoxycholate (Sigma-Aldrich, catalog number: D6750)
- Sucrose, M = 342.3 g/mol (Sigma-Aldrich, catalog number: S0383)
- Trizma® base, M = 121.14 g/mol (Sigma-Aldrich, catalog number: T6066)
- Tween-20 (J.T. Baker, catalog number: 7374)
- Western Blotting Luminol Reagent (Santa-Cruz Biotechnology, catalog number: sc-2048), storage at 4 °C
- Milk powder (Carl Roth, catalog number: T145-1)
- EDTA disodium salt (J.T. Baker, catalog number: 6381-92-6)
- SDS (Sigma-Aldrich, catalog number: L4509)
- Methanol (Carl Roth, catalog number: 4627.4)
- HCl (Carl Roth, catalog number: 4625.1)
- Xylazin 20 mg/ml (Serumwerke Bernburg AG, catalog number: Ch.B: 01117)
- Ketamin 10% (WDT, Ch.B: J3218-03)
- Liquid N2
- Alcohol
- Acetone
- Homogenization Buffer (see Recipes)
- Isolation buffer (see Recipes)
- RIPA buffer (see Recipes)
- 1x TBS (see Recipes)
- TBST (see Recipes)
- Blocking Buffer (see Recipes)
- 1 M Sucrose (see Recipes)
- 1 M Tris-HCl (see Recipes)
- 0.5 M EDTA (see Recipes)
- 1 M Mannitol (see Recipes)
- 1 M MgCl2 (see Recipes)
- 0.5 M MOPS (see Recipes)
- 100 mM EGTA (see Recipes)
- 1 M K2HPO4 (see Recipes)
- 50 mM PMSF (see Recipes)
- Antibodies
Equipment
- Pipettes
- Dissection Instruments
- Centrifuge (Eppendorf AG, model: 5415R)
- Centrifuge Sorvall superspeed (Herolab GmbH Laborgeräte, model: Sorvall RC5CPlus)
- Chemiluminescence Imaging System (Vilber, model: Fusion Solo S)
- Multi-Sample Osmometer (Advanced Instruments, model: 2020)
- Nanodrop (Thermo Fisher Scientific, Life technologiesTM, model: 2000C)
- Overhead shaker GFL 3025 (Laborgeräte München)
- Mini-Blot-Schüttler, 3-D (VWR, Lab Services GmbH)
- Spectrophotometer (Harvard Bioscience, GeneQuant, catalog number: 80-2109-02)
- Jun Air compressor 87R-15B with manometer (-1 to +0.6bar) (JUN-AIR Gast Manufacturing, Inc, USA)
Software
- Excel (Microsoft Cooperation, Redmond, USA)
- Maschine Fusion SL, Vilber Lourmat Software: FusionCapt Advance SL4 16.08a
Procedure
文章信息
版权信息
© 2019 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Micakovic, T., Banczyk, W. Z., Clark, E., Kränzlin, B., Peters, J. and Hoffmann, S. C. (2019). Isolation of Pure Mitochondria from Rat Kidneys and Western Blot of Mitochondrial Respiratory Chain Complexes. Bio-protocol 9(19): e3379. DOI: 10.21769/BioProtoc.3379.
分类
细胞生物学 > 细胞器分离 > 线粒体
分子生物学 > 蛋白质 > 细胞间转移
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link