- EN - English
- CN - 中文
A Cell Culture Model that Mimics Physiological Tissue Oxygenation Using Oxygen-permeable Membranes
一种利用透氧膜模拟生理组织氧合的细胞培养模式
发布: 2019年09月20日第9卷第18期 DOI: 10.21769/BioProtoc.3371 浏览次数: 7259
评审: Gal HaimovichMarzia Di DonatoPhilipp Wörsdörfer
Abstract
Dissolved oxygen and its availability to cells in culture is an overlooked variable which can have significant consequences on experimental research outcomes, including reproducibility. Oxygen sensing pathways play key roles in cell growth and behavior and pericellular oxygen levels should be controlled when establishing in vitro models. Standard cell culture techniques do not have adequate control over pericellular oxygen levels. Slow diffusion through culture media limits the precision of oxygen delivery to cells, making it difficult to accurately reproduce in vivo-like oxygen conditions. Furthermore, different types of cells consume oxygen at varying rates and this can be affected by the density of growing cells. Here, we describe a novel in vitro system that utilizes hypoxic chambers and oxygen-permeable culture dishes to control pericellular oxygen levels and provide rapid oxygen delivery to adherent cells. This procedure is particularly relevant for protocols studying effects of rapid oxygen changes or intermittent hypoxia on cellular behavior. The system is inexpensive and easily assembled without highly specialized equipment.
Keywords: Cell culture (细胞培养)Background
Oxygen availability can impact on many signaling pathways and small changes to the level of pericellular oxygen can alter growth rate, metabolism, free radical production, and general cell behavior (Place et al., 2017; Bordt, 2018). Therefore, to preserve normal cellular function, oxygen levels need to be maintained within a relatively narrow ‘normoxic’ range. This applies both to cells in vivo and to cell lines used in cell culture (Hunyor and Cook, 2018).
In most cell culture models, experimental ‘normoxic’ values are based on the oxygen levels supplied in cell culture incubators. In dry air at sea level, atmospheric oxygen is ~20.9% v/v (Hunyor and Cook, 2018). In standard humidified cell culture incubators maintained at 5% CO2, oxygen levels are equal to 18.6% O2 (v/v), equivalent to an oxygen tension or oxygen partial pressure (pO2) of 138 mmHg (Wenger et al., 2015). However, cells are maintained at much lower pO2 levels in vivo (Carreau et al., 2011). Under normal ventilatory conditions, pO2 in the arterial blood ranges from 80-100 mmHg (10.8-13.5% O2 v/v) and this is further lowered as oxygen molecules diffuse through tissue and are consumed by cells (Carreau et al., 2011). Moreover, different tissues have unique ranges of physiological normoxia, depending on the specific oxygen demands of individual tissues. Using a single oxygen concentration to represent physiological normoxia for a range of cell types is not representative of the in vivo environment (Carreau et al., 2011). When studying pathological oxygen conditions (such as in ischemia or in tumor hypoxia) cells should be exposed to oxygen tensions that appropriately model the specific pathological state of the tissue. In brain ischemia, pO2 levels can decrease below 10 mmHg (1% v/v) (Hoffman et al., 1999). In tumors, pO2 levels can range from 15 mmHg (2.1% v/v) to as low as 2 mmHg (0.3% v/v) and this can also depend on the tissue of origin (Byrne et al., 2014).
The concentration of oxygen used to represent hypoxia is important because oxygen-sensing proteins have optimal pO2 ranges for which they operate. Hypoxia inducible factor (HIF) is a transcription factor activated by low oxygen. HIF is thought to control the expression of up to 1,000 genes and it has important roles in health and disease including embryogenesis (Dunwoodie, 2009), angiogenesis (Cook and Figg, 2010), cancer (Cook et al., 2009), diabetes (Thangarajah et al., 2010), inflammation and immune function (Palazon et al., 2014). HIF-1α and HIF-2α have been found to be stabilized under different oxygen tensions (Holmquist-Mengelbier et al., 2006; Lin et al., 2011). In neuroblastoma, HIF-2α is stabilized in 5% O2 and can be strongly expressed even in well-vascularized areas, while HIF-1α is stabilized below 1% O2 in extremely hypoxic regions of the tumor (Holmquist-Mengelbier et al., 2006). A small change in oxygen levels can therefore alter the balance of HIF-1α and HIF-2α, which affects what genes are expressed and ultimately how the cells behave. As we build better in vitro systems to accurately model in vivo conditions, oxygen levels are a key experimental variable to consider.
A common method used to modulate oxygen conditions in cell culture involves the use of a CO2 incubator with variable oxygen control (Wu and Yotnda, 2011). The major drawback of this method when using traditional cell culture techniques is that long exposure periods are required for the oxygen present in the gas phase to diffuse to cells attached at the bottom of the culture dish. Depending on the volume and depth of media, this can take hours due to poor oxygen diffusivity in aqueous media (Allen et al., 2001). Oxygen conditions at the pericellular level therefore do not immediately match the oxygen conditions supplied in the incubator air. One method to counteract this is to pre-equilibrate media/reagents to the desired pO2 levels prior to culturing. The major issue with this is that unless the cell culture is handled within a closed compartment (such as in an airtight glove box), oxygen from the ambient air is constantly diffusing into media and reagents, preventing the precise control over the oxygen concentrations being delivered to cells (Byrne et al., 2014). Many laboratories are not readily equipped with oxygen-controlled incubators or glove boxes due to their high costs.
As a more cost-effective alternative, we have designed a cell culture system that utilizes commercially available oxygen-permeable cultureware. The culture dishes are composed of a thin membrane surface that is highly permeable to oxygen. Cells adhere to the membrane and are covered in media as with normal cell culture methods. However, due to the extremely high oxygen permeability of the membranes, cells have access to oxygen almost instantly from ambient air through the membrane rather than through the media (Figure 1). This enables faster oxygen equilibration rates to be achieved at the cellular level. These fast rates of oxygen cycling therefore make this model particularly useful for protocols aimed at studying rapid changes in oxygenation or intermittent hypoxia, relevant to diseases such as cancer (Michiels et al., 2016), obstructive sleep apnea (Minoves et al., 2017; Martinez et al., 2019), cardiovascular disease and ischemia (Savla et al., 2018).
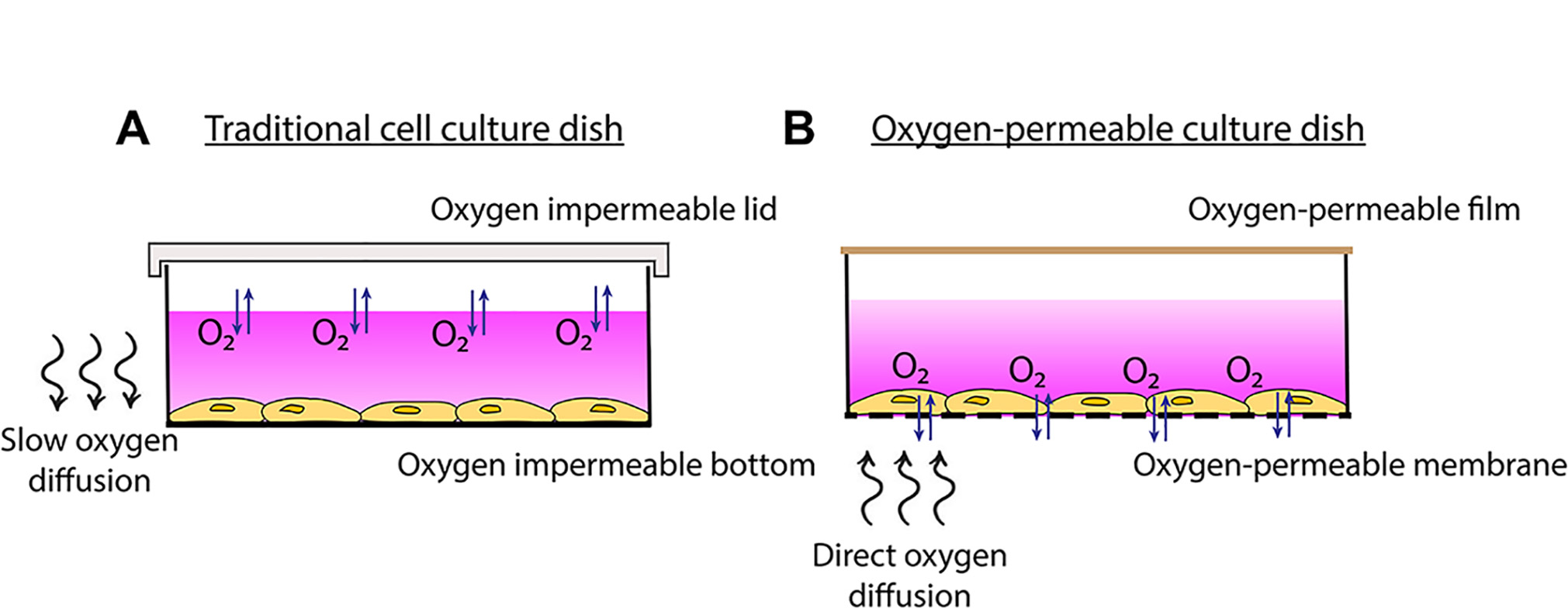
Figure 1. Traditional cell culture dish vs. oxygen-permeable culture dish. A. Traditional cell culture dishes are impermeable to oxygen so more time is required for oxygen to diffuse through the media to the cells. B. Plating cells onto oxygen-permeable membranes enables gas-phase oxygen to be sourced directly through the membrane.
Materials and Reagents
- T75 flask
- Pipette tips
- 15 ml sterile tube
- 1.5 ml microtubes
- Oxygen-permeable culture plate (commercially available plates are shown in Table 1). This protocol uses a 2-well slide plate from Coy Lab Products
Table 1. List of commercially available oxygen-permeable membranes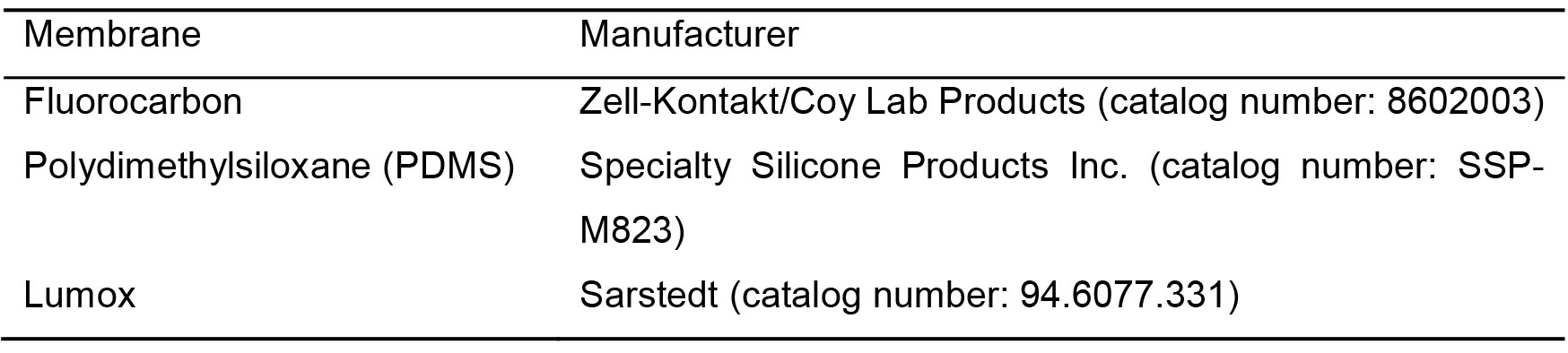
- AeraSealTM film sterile (Sigma-Aldrich, catalog number: A9224-50EA)
- Acrylic hypoxic chamber (Custom-made)
- Clear Acrylic Round Cylinder Display Riser (Plymor, available through Amazon, USA)
- 1 cm thick flat piece of acrylic (Plymor, available through Amazon, USA)
- Tubing adaptors
- Silicone grease
- Epoxy or silicone glue
- BlutackTM
- McCoy’s modified media (Thermo Fisher, Gibco, catalog number: 36600-021)
- DMEM/F12 media (Thermo Fisher, Gibco, catalog number: 11320-033)
- DMEM media (Thermo Fisher, Gibco, catalog number: 10313-039)
- Foetal bovine serum (Thermo Fisher, Gibco, Australian FBS, catalog number: 26140-079)
- Penicillin-Streptomycin (Thermo Fisher, Gibco, catalog number: 15070-063)
- GlutaMAX (Thermo Fisher, Gibco, catalog number: 35050-061)
- Dulbecco’s phosphate buffered saline (Gibco, catalog number: 14190-144)
- TrypLE (Thermo Fisher, Gibco, catalog number: 12605-028)
- RNeasy Mini Kit (Qiagen, catalog number: 74104)
- Tris-HCl (Sigma-Aldrich, catalog number: T3253)
- NaCl (Omnipur, catalog number: 7710)
- EDTA (Sigma-Aldrich, catalog number: EDS)
- Na4P2O7 (Chem Supply, catalog number: TA045)
- SDS (Sigma-Aldrich, catalog number: L3771)
- Sodium deoxycholate (Sigma-Aldrich, catalog number: D6750)
- Triton X (VWR, catalog number: ICNA04807426)
- Glycerol (Sigma-Aldrich, catalog number: G5516)
- HEPES (Sigma-Aldrich, catalog number: H3375)
- KCl (Sigma-Aldrich, catalog number: P9333)
- Nonidet-P40 Substitute solution (Sigma-Aldrich, catalog number: 98379)
- DTT (Pierce, Thermo Fisher Scientific, catalog number: 20291)
- RIPA lysis buffer (see Recipes)
- Cytoplasmic extraction buffer (see Recipes)
- Nuclear extraction buffer (see Recipes)
Equipment
- Laminar flow hood
- Standard humidified cell culture incubator maintained at 5% CO2
- Water bath (37 °C)
- Hemocytometer
- Microscope (Zeiss Primovert, 5x and 10x objective lenses)
- Centrifuge (Eppendorf, model: 5810R)
- Gas blender (3 channel GB100, MCQ Instruments, version GB1E43120240)
- N2, CO2 and O2 gas sources
- Pre-mixed gas cannisters (BOC, catalog number: CCS15388E)
- Tubing (provided by MCQ instruments, or can be purchased from Saint-Gobain T3)
- Clamp stand
- Profiling oxygen microsensor (PM-PSt7, PreSens) connected to a compact oxygen transmitter (OXY-1 ST, PreSens)
- Microcentrifuge (Thermo Fisher Scientific, Heraeus Fresco 21 [4 °C])
Software
- MCQ Gas Mixture Creator PRO (MCQ Instruments, version 1)
- PreSens Measurement Studio 2
- Microsoft Excel
- GraphPad Prism
Procedure
文章信息
版权信息
© 2019 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Martinez, C., Cistulli, P. A. and Cook, K. M. (2019). A Cell Culture Model that Mimics Physiological Tissue Oxygenation Using Oxygen-permeable Membranes. Bio-protocol 9(18): e3371. DOI: 10.21769/BioProtoc.3371.
分类
癌症生物学 > 通用技术 > 肿瘤微环境
癌症生物学 > 微环境 > 低氧
细胞生物学 > 细胞分离和培养 > 单层培养
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link