- EN - English
- CN - 中文
A Widely Applicable Urea-based Fluorescent/Colorimetric mRNA in situ Hybridization Protocol
一种广泛应用的尿素荧光/比色mrna原位杂交技术
发布: 2019年09月05日第9卷第17期 DOI: 10.21769/BioProtoc.3360 浏览次数: 6859
评审: Gal HaimovichVarpu MarjomakiAnca Flavia Savulescu
Abstract
In situ hybridization methods are routinely employed to detect nucleic acid sequences, allowing to localize gene expression or to study chromosomal organization in their native context. These methods rely on the pairwise binding of a labeled probe to the target endogenous nucleic acid sequence–the hybridization step, followed by detection of annealed sequences by means of fluorescent or colorimetric reactions. Successful hybridization requires permeabilization of tissues, followed by denaturation of nucleic acids strands, which is usually carried out in a formamide-based buffer and at high temperatures. Such reaction conditions, besides posing a health hazard (both concerning manipulation and waste disposal), can be excessively harsh for the delicate tissues of some species or developmental stages. We detail here an alternative method for in situ hybridization, where the toxic formamide is replaced with a urea solution. This substitution improved both tissues preservation and signal-to-noise detection, in several animal species. The protocol described here, originally developed for the hydrozoan jellyfish Clytia hemisphaerica, provides guidelines for adapting formamide-based traditional protocols to the urea variant. Urea-based protocols have already been successfully applied to diverse invertebrate and vertebrate species, showing the ease of such a modification, and providing the scientific community with a promising, safer and versatile tool.
Keywords: mRNA in situ hybridization (RNA原位杂交)Background
The pairwise complementary binding of single strand nucleic acid sequences inspired the development of a powerful technique, in situ hybridization, which allows researchers to visualize the location of DNA or RNA strands within their cellular and tissue contexts (Pardue and Gall, 1969). Numerous variants have since been developed, differing for the labeling of the exogenous complementary probes (e.g., radioactive or hapten-based), in the type of samples (e.g., for whole embryos or tissue sections) and in the detection method (e.g., fluorescent or colorimetric signals).
Precise annealing of probes depends on the accessibility of the target sequences, achieved through appropriate permeabilization of tissues and cell membranes (which allows penetration of reagents), and denaturation of nucleic acid strands (which exposes the complementary target sequences). Achieving adequate sensitivity of detection, specificity of annealing, and preservation of sample morphology often requires a time-consuming optimization of reaction conditions. Efficient denaturation and annealing of nucleic acids strands are usually obtained by means of elevated temperatures and the addition of a denaturing reagent in the hybridization buffer.
Reaction temperatures should ideally be adapted to the base composition of the sequences of interest: hybridization temperatures are about 25 °C below denaturation temperatures (Tm; Marmur and Doty, 1961). 50-70% formamide is usually employed as the denaturing reagent, this choice remaining largely unchallenged since the ‘80s–with few exceptions, such as the smFISH (single-molecule Fluorescence In Situ Hybridization) approach where 10-15% formamide is used (Haimovich and Gerst, 2018).
Elevated temperatures increase evaporation of the hybridization buffer, compounding the health risk already posed by formamide, an irritating, embryotoxic and teratogenic solvent (Gleich, 1974; Stula and Krauss, 1977; Merkle and Zeller, 1980; Kennedy and Short, 1986; Fail et al., 1998; George et al., 2000 and 2002; see also Sinigaglia et al., 2018 for further information). Hybridizing samples and waste need to be appropriately handled (CICAD 31), thus limiting the application of standard formamide-based protocols in sensitive contexts, such as pregnancy or in classrooms.
In the early years, a diversity of solvents was found to effectively destabilize nucleic acid strands, with urea being particularly efficient (Herskovits, 1963). Indeed, urea and formamide have similar properties, and are employed equivalently in a number of applications, such as fluorescent in situ hybridization on bacteria (Fontenete et al., 2016), protein denaturation, and clearing agents for imaging (reviewed in Azaripour et al., 2016). Urea can effectively replace formamide in Northern and Southern blot experiments, in particular at concentrations in the 2 M-4 M range (Simard et al., 2001). The mechanism of action of urea is still relatively poorly understood. Urea lowers the Tm of DNA of about 2 °C per mole of urea (Hutton, 1977), and is thus slightly less efficient than formamide, which achieves a 2.4-2.9 °C reduction per mole. Urea molecules bind weakly to RNA, disrupting its base-pair interactions and ultimately destabilizing the structure of RNA molecules (Herskovits and Bowen, 1974; Priyakumar et al., 2009; Lambert and Draper, 2012).
Urea, in powder or crystal form, is water soluble, and 8 M stock solutions can be readily prepared at 20 °C. Solutions tend to be viscous (Hutton, 1977), which might explain why high concentrations of urea are less effective (Simard et al., 2001). Such increased viscosity demands care during the hybridization step of the in situ protocol, to ensure that samples are efficiently immersed, and evaporation minimized.
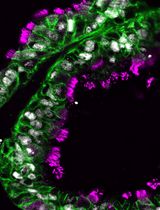
The urea-based protocol presented here has been shown to be more efficient than the standard formamide-based ones in diverse metazoan species and at different developmental stages (see Sinigaglia et al., 2018). Detection sensitivity was improved, allowing visualization of unsuspectedly complex patterns of gene expression (Sinigaglia et al., 2018). This increased sensitivity of detection might be explained by an additional permeabilizing action of urea on tissues (Lim et al., 2009; Huang et al., 2011), likely due to increased osmotic pressure within cells (reviewed in Tainaka et al., 2016). The hyperhydration of tissues might also partially account for the improved morphologies of delicate samples, shown in the original research paper (Sinigaglia et al., 2018).
The protocol detailed here was originally developed for the hydrozoan medusa Clytia hemisphaerica (Quiroga Artigas et al., 2018; Sinigaglia et al., 2018, Leclère et al., 2019). Following those guidelines, urea-alternative protocols have already been developed for a diversity of metazoan species and developmental stages, including priapulid and brachiozoan embryos (Thiel et al., 2017; Sinigaglia et al., 2018), the acoel Hofstenia miamia (L. Ricci, personal communication), the scyphozoan jellyfish Aurelia aurita (M. Manuel and T. Condamine, personal communication), and in the paddlefish (M. Minarik, personal communication). Substituting formamide with urea has also been applied to DNA-FISH on mouse oocytes (Manil-Segalen et al., 2018), further showing the versatility of this approach.
Such versatility stems from the simplicity of the key implementation, the substitution of formamide with an equal volume of urea solution (at a final concentration of 4 M). When adapting previous protocol to novel species or tissues, it is recommended to start by simply switching formamide to urea, leaving the rest of the procedure unchanged. Guidelines for eventual troubleshooting are also provided, offering general recommendations for successful in situ hybridization.
Materials and Reagents
- Materials and reagents used in multiple steps
- Gloves
- RNase free tubes (e.g., 1.5 ml Eppendorf tubes, or equivalent)
- In situ baskets (Sample container, as in Figure 1A)
They could be either the Netwell baskets (Corning), or homemade baskets constructed from nylon mesh and microcentrifuge tubes, as explained in Sive et al. (2007). Baskets size depends on the type of sample; they usually fit within the wells of a 6-, 12- or 24-well plate. Performing in situ hybridization in baskets (instead of tubes) is recommended:- To reduce loss and damages to samples, due to pipetting.
- To accelerate exchange of solutions (baskets can be simply transferred with the help of forceps to a multiwell plate with the new solution (Figure 1B)–in this case tap gently the basket against the walls of the well to ensure removal of older solution, then gently immerse it in the new well).
- Baskets can be recycled multiple times, after being thoroughly washed (e.g., rinse with MilliQ H2O, then immerse in a 1 M NaOH solution to neutralize RNases, and rinse several times with RNase-free MilliQ H2O–simply placing them in a beaker on a shaker will assure thorough swirling).

Figure 1. Sample carrier. A. Example of three baskets with mesh-bottom. B. A 24-well plate used for in situ hybridization, with four baskets. C. The same plate, closed and wrapped in plastic film to avoid evaporation during hybridization step.
- Multiwell plate (For fitting in situ baskets, as in Figure 1B); (e.g., 24-well cell culture plate, sterile, with lid, from CellStar (Greiner Bio-one, catalog number: 662-160))
- Ice and ice container
- MilliQ H2O (Storage: RT)
- Tween-20 (Sigma-Aldrich, catalog number: P1379), storage: RT (Mild detergent. Given its high viscosity, it is recommended to prepare a 10% working solution)
- Na2HPO4·7H2O (Sigma-Aldrich, catalog number: 431478), storage: RT (For preparing 10X PBS solution)
- NaCl (Sigma-Aldrich, catalog number: S9888), storage: RT (For preparing 10X PBS solution)
- KCl (Sigma-Aldrich, catalog number: P9333), storage: RT (For preparing 10X PBS solution)
- KH2PO4 (Sigma-Aldrich, catalog number: P9791), storage: RT (For preparing 10X PBS solution)
- 10x PBS (Phosphate-Buffered Saline) pH 7.4 (see Recipes, or from tablets from Calbiochem, catalog number: 524650), storage: RT (Helps maintaining a constant pH)
- Fixation
- 37% formaldehyde (Sigma-Aldrich, catalog number: 252549), storage: RT (Crosslinking)
- 8% glutaraldehyde (Electron Microscopy Sciences, catalog number: 16020), storage: 4 °C (Crosslinking)
- EGTA (Ethylene glycol-bis(2-aminoethylether)-N,N,N',N'-tetraacetic acid) (Sigma-Aldrich, catalog number: E3889), storage: RT [Calcium/magnesium chelator, which reduces enzyme activity (such as RNase); reagent of HEM buffer]
- HEPES (Sigma-Aldrich, catalog number: H4034), storage: RT (Reagent of HEM buffer)
- MgSO4 (Sigma-Aldrich, catalog number: M7506), storage: RT (Reagent of HEM buffer)
- Tween-20 (Sigma-Aldrich, catalog number: P1379), storage: RT
- Methanol (e.g., Sigma-Aldrich, catalog number: 322415), storage: RT
- 1x PBS (see Recipes)
- 1x PBST (see Recipes)
- CISH fixation buffer (see Recipes)
- FISH fixation buffer (see Recipes)
- Dehydration buffer (see Recipes)
- Rehydration buffer (see Recipes)
- Re-fixation buffer (see Recipes)
- Acetylation
- TEA (Triethylamine) (Sigma-Aldrich, catalog number: T0886), storage: RT (For reducing the non-specific binding of negatively-charged probe to tissues)
- Acetic anhydride (Sigma-Aldrich, catalog number: A6404), storage: 4 °C (For reducing the non-specific binding of negatively-charged probe to tissues)
- 1x PBST (see Recipes)
- 10x TEA stock solution (see Recipes)
- 0.1 M TEA (see Recipes)
- 0.25% Acetic anhydride in 0.1 M TEA (see Recipes)
- Permeabilization
- Proteinase K (Sigma-Aldrich, catalog number: P4850), storage: 4 °C or -20 °C (Digests proteins, including nucleases. Optimum pH is 7.5-9.0)
- Glycine (Sigma-Aldrich, catalog number: G8898), storage: RT (For neutralizing the proteinase K)
- 1x PBST (see Recipes)
- Hybridization
- Plastic film (For wrapping sample container during hybridization step)
- Urea (Sigma-Aldrich, catalog number: U5378), storage: RT (Denaturing agent)
- Dextran sulfate sodium salt, Mr ~200''00 (Sigma-Aldrich, catalog number: 67578-25G), storage: RT [Increases molecular crowding and can therefore accelerate the kinetics of hybridization (locally increases probe concentration)]
- tRNA, from baker’s yeast (Sigma-Aldrich, catalog number: 10109509001), storage: -20 °C (Blocks non-specific probe binding)
- Salmon sperm (Thermo Fisher, catalog number: 15632011), storage: -20 °C (Blocks non-specific probe binding)
- Heparin sodium (Thermo Fisher, catalog number: 10239840), storage: -20 °C (Helps to reduce background, by binding to proteins that bind to DNA)
- 20% SDS (Sodium Dodecyl Sulfate) (Fisher, catalog number: BP131), storage: RT (Detergent. Permeabilizes membranes, facilitating probe distribution)
- NaCl (Sigma-Aldrich, catalog number: S9888), storage: RT (Salt. For preparing 20x SSC stock buffer, see Recipes)
- Trisodium citrate (Na3C6H5O7) (For preparing 20x SSC stock buffer, see Recipes)
- Tween-20 (Sigma-Aldrich, catalog number: P1379), storage: RT
- 10x DIG labeling mix RNA (Roche, catalog number: 11277073910), storage: -20 °C (For probe synthesis)
- RNasin Ribonuclease Inhibitor (Promega, catalog number: N2111 or N2511), storage: -20 °C (For probe synthesis)
- DTT (Promega, catalog number: P1171), storage: -20 °C (For probe synthesis)
- Transcription Optimized 5x Buffer (Promega, catalog number: P1181), storage: -20 °C (For probe synthesis)
- T7/SP6/T3 RNA polymerase (Promega, catalogue number, respectively: P2075/ P1085/ P2083), storage: -20 °C (For probe synthesis)
- RQ1 RNase-free DNase (Promega, catalog number: M6101), storage: -20 °C (For probe synthesis)
- Illustra ProbeQuant G-50 Micro Columns (GE Healthcare, catalog number: 28-9034-08) (For probe synthesis)
- 1x PBST (see Recipes)
- 8 M Urea stock solution (see Recipes)
- 20x SSC (Sodium Saline Citrate or Standard Saline Citrate) (see Recipes, or from Sigma-Aldrich, catalog number: S6639), storage: RT [Controls stringency (annealing of probe to target)]
- Wash I (see Recipes)
- Wash II (see Recipes)
- Wash III (see Recipes)
- Urea-based 100% Hybridization Buffer (see Recipes)
- Urea-based 50% Hybridization Buffer (see Recipes)
- Antibody incubation
- Anti-Digoxigenin-AP, Fab fragments (Roche Applied Sciences, Sigma-Aldrich, catalog number: 11093274910), storage: -20 °C [Recognizes the DIG hapten, conjugated to Alkaline Phosphatase (AP)]
- Anti-Digoxigenin-POD, Fab fragments (Roche Applied Sciences, Sigma-Aldrich, catalog number: 11207733910), storage: -20 °C [Recognizes the DIG hapten, conjugated to horseradish Peroxidase (POD)]
- Blocking reagent (Sigma-Aldrich, catalog number: 11096176001), storage: 20-25 °C (To decrease non-specific binging of antibodies)
- Maleic acid (Sigma Aldrich, catalog number: M0375), storage: RT (For preparing MAB)
- NaCl (Sigma-Aldrich, catalog number: S9888), storage: RT (Salt. For preparing MAB)
- NaOH (Sigma-Aldrich, catalog number: 221465), storage: RT (For preparing MAB)
- 10x blocking reagent stock solution (see Recipes), storage: -20 °C
- 10x MAB (Maleic Acid Buffer) stock solution (see Recipes) (For diluting blocking buffer, used after SSC washes)
- 1x MABT buffer (see Recipes)
- 10x blocking reagent stock solution (see Recipes), storage: -20 °C
- 1x Blocking buffer (see Recipes)
- Detection
- Aluminum foil (For protecting samples during staining step)
- BCIP (5-Bromo-4-Chloro-3-Indolyl-Phosphate)/NBT (Nitro Blue Tetrazolium) Color Development Substrate (Promega, catalog number: S3771), storage: 4 °C or -20 °C [Substrate of alkaline phosphatase which generates a dark (blue/violet) precipitate]
- NaCl (Sigma-Aldrich, catalog number: S9888), storage: RT (Salt. Component of NTMT buffer)
- MgCl2 (Sigma-Aldrich, catalog number: M8266), storage: RT (Component of NTMT buffer, MgCl2 is a necessary cofactor for alkaline phosphatase)
- Tris-HCl (Tris hydrochloride) (Sigma, catalog number: T3253), storage: RT (pH buffering component of NTMT)
- Tween-20 (Sigma-Aldrich, catalog number: P1379), storage: RT
- Levamisole (Agilent Dako, catalog number: X302130-2), storage: 2-8 °C (Alkaline Phosphatase inhibitor)
- TSA Plus Fluorescence Amplification kit Cyanine 3/5 (Perkin Elmer, Waltham, MA; catalog number: NEL752001KT), storage: 4 °C [Tyramide Signal Amplification (TSA) kit uses Horseradish Peroxidase (HRP or Horseradish POD) to catalyze the deposition of a fluorophore-labeled radicals]
- H2O2 (Sigma-Aldrich, catalog number: 516813), storage: 4 °C (Substrate for horseradish peroxidase)
- Filters for syringe (Millex-GS, catalog number: SLGS033SS) (For filtering NTMT buffer, in order to avoid precipitates)
- Disposable syringes (e.g., 50 ml from Thermo Scientific, catalog number: S7510-50) (For filtering NTMT buffer, in order to avoid precipitates)
- 5 M NaCl stock solution (For preparing NTMT buffer, see Recipes), storage: RT
- 1 M Tris-HCl, pH 9.5 stock solution (For preparing NTMT buffer, see Recipes), storage: RT
- 1 M MgCl2 stock solution (For preparing NTMT buffer, see Recipes), storage: RT
- NTMT buffer (see Recipes)
- NTMT minus buffer (see Recipes)
- NBT/BCIP in NTMT buffer (see Recipes)
- Storage & Mounting
- Glycerol (Sigma-Aldrich, catalog number: G5516), storage: RT (Mounting medium)
- Citifluor-AF1 (Citifluor, catalog number: AF1-100), storage: RT [Mounting medium for fluorescent samples (on-hardening antifading/anti-bleaching)]
- Slide (e.g., 76 x 26 mm, with frosted end (practical for labeling), from Knittel Glass (Mounting)
- Coverslide (e.g., 20 x 20 mm, thickness 1, from Knittel Glass) (Mounting)
- Nailpolish (For sealing mounted specimens; any commercial nailpolish can be used, however prefer a transparent one, and test first on lesser important samples, in case it contains too much solvent–which might damage samples)
Equipment
Note: No specific brand/model is provided; this equipment is commonly available in laboratories.
- Pipettes
- Heating block (For probe denaturation, e.g., featuring a temperature range 10-100 °C)
- Metallic forceps (For transferring baskets, e.g., from Fine Science Tools)
- Waterbath/Hybridization oven (Hybridization and stringent washes)
- Glass bottles with high-temperature resistant screw caps (e.g., 250 ml bottle from Fisherbrand (catalogue number: FB-800-250); 500 ml bottle from VWR [borosilicate 3.3; catalogue number: 215-1594); 1000 ml bottle from Scott Duran (Original GL 45 series)]
- pH meter (For adjusting pH of solutions)
Procedure
文章信息
版权信息
© 2019 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Sinigaglia, C. (2019). A Widely Applicable Urea-based Fluorescent/Colorimetric mRNA in situ Hybridization Protocol. Bio-protocol 9(17): e3360. DOI: 10.21769/BioProtoc.3360.
分类
发育生物学 > 形态建成 > 器官形成
细胞生物学 > 细胞成像 > 固定组织成像
分子生物学 > RNA > RNA 标记
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link