- EN - English
- CN - 中文
Optimized Protocol for the Incorporation of FDAA (HADA Labeling) for in situ Labeling of Peptidoglycan
FDAA用于肽聚糖原位标记的优选方案
发布: 2019年08月05日第9卷第15期 DOI: 10.21769/BioProtoc.3316 浏览次数: 9105
评审: Andrea PuharAksiniya AsenovaYoko Eguchi

相关实验方案

蓝藻Synechococcus elongatus PCC 7942中多拷贝染色体的直接可视化观察
Ryudo Ohbayashi [...] Satoru Watanabe
2018年08月05日 6912 阅读
Abstract
The essential peptidoglycan (PG) layer surrounds the cytoplasmic membrane in nearly all bacteria. It is needed to maintain the shape of the cell and protect it from lysis due to high turgor. Growth of the PG layer is a complex process that involves the activities of PG synthases and hydrolases during elongation and cell division. PG growth sites can be labeled by the recently developed fluorescent D-amino acid (FDAA) probes in a range of different bacteria. FDAAs are incorporated into PG by DD-transpeptidases (Penicillin-binding proteins, PBPs) or, if present, LD-transpeptidase (LDTs). Long-pulse in situ labeling of E. coli cells with the FDAA 7-hydroxycoumarincarbonylamino-D-alanine (HADA) is expected to result in a uniform label at the side wall of cells and enhanced label at cell division sites due to the intense PG synthesis. However, we observed reduced label at mid-cell when labeling E. coli cells with HADA. We reasoned that probe incorporated at cell division sites may be removed by PG hydrolases and modified the labeling protocol to better preserve PG-incorporated HADA for fluorescence microscopy. Here, we report the optimized HADA-labeling protocol by which cells retain an enhanced HADA signal at the division septum.
Keywords: Fluorescence D-amino acid (FDAA) (荧光D-氨基酸)Background
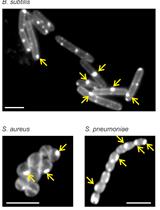
The peptidoglycan (PG) sacculus is a mesh-like, essential macromolecule that encases the cytoplasmic membrane in most bacteria. It is composed of glycan chains made of alternating β-1, 4-linked N-acetylglucosamine and N-acetylmuramic acid residues connected via short peptides and is required to maintain the shape and osmotic stability of a bacterial cell (Typas et al., 2011). Growing and dividing bacteria synthesize new PG and incorporate it into their PG sacculus, and these processes are targeted by important antibiotics such as the β-lactams and glycopeptides. To visualize the growth sites on the PG sacculus Miguel de Pedro developed an elegant method in which D-cysteine (D-Cys) is first homogeneously incorporated into PG of growing bacteria (by then unknown enzymes), followed by a chase period in the absence of D-Cys. PG sacculi were isolated, followed by the selective biotinylation of D-Cys residues and the visualization of biotin with nano-gold labeled antibodies by electron microscopy (de Pedro et al., 1997). PG growth sites were characterized by regions of reduced label (when D-Cys became 'diluted' upon incorporation of new, label free PG during the chase) or without label (a zone of exclusively new PG). Although this method was crucial to determine the modes of PG segregation in model species such as Escherichia coli (de Pedro et al., 1997) and Caulobacter crescentus (Aaron et al., 2007), it was relatively cumbersome and used mainly in specialist laboratories. Over the last years, Erkin Kuru, Michael VanNieuwenhze and Yves Brun developed an ever-increasing palette of fluorescent D-amino acid (FDAA) probes that covalently label PG in cells and can therefore be used to visualize PG by fluorescence microscopy (Kuru et al., 2012 and 2015; Hsu et al., 2017). New 'rotor probes' of this series, rFDAA, produce fluorescence only upon incorporation into PG, enabling real-time visualization of probe incorporation and making it unnecessary to wash cells after the labeling procedure (Hsu et al., 2019). Presumably FDAAs are incorporated into PG by PBPs and LDTs, as are other D-amino acids (Cava et al., 2011; Lupoli et al., 2011). FDAAs efficiently label sites of active PG synthesis and have therefore been successfully used to track PG synthesis sites in different bacterial species (Kuru et al., 2012; Radkov et al., 2018). They are easy to use and reliably label the PG in many bacteria and they have contributed to major discoveries in a variety of bacterial species (Radkov et al., 2018), for example, the discovery of PG in Chlamydia trachomatis and its visualization (Liechti et al., 2014 and 2016), the tracking of septal PG synthesis dynamics in B. subtilis (Bisson-Filho et al., 2017) and the visualization of preseptal PG synthesis in E. coli (Pazos et al., 2018).
We recently showed that copper(II) inhibits LD-TPases in vivo and in vitro (Peters et al., 2018). Within this study we performed long-pulse in situ labeling of E. coli cells with the FDAA 7-hydroxycoumarincarbonylamino-D-alanine (HADA), which was previously shown to uniformly label cells (Kuru et al., 2012 and 2015). Indeed, using the published procedure wild-type cells showed homogeneous label along the lateral wall but, unexpectedly, constricting cells showed a reduced signal at the septum. No signal could be detected in a strain lacking all six LD-TPases (Peters et al., 2018). During cell growth PG synthesis takes place at the division septum due to the activities of PG synthases (PBP1B and PBP3) (de Pedro et al., 1997; Bertsche et al., 2006). We reasoned that the HADA signal at mid-cell was low despite the enhanced PG synthesis because the incorporated HADA might be removed by PG hydrolases during the time the cells were harvested and washed at neutral pH, resulting in the loss of label at the division septum. Indeed, labeling of chlamydial PG with fluorescent dipeptide required the inactivation of DD-carboxypeptidases by amplicillin (Liechti et al., 2014). Here we optimized the long-pulse HADA labeling protocol to reduce the potential loss of HADA label by PG hydrolases. Specifically, we stopped cell growth and label incorporation by adding sodium citrate buffer pH 2.25 to the growing cells and washed the cells once with sodium citrate buffer at pH 3.0, followed by two washing steps with phosphate-buffered saline (PBS, pH 7.4), prior to processing the samples for fluorescence microscopy. With this procedure cells of wild-type and several mutants were homogeneously labeled at the side-wall and had enhanced signal at the septum of constricting cells. We conclude that the rapid incubation and washes of cells at acidic pH preserves the HADA label by preventing the removal of incorporated HADA by PG hydrolases (Amanuma and Strominger, 1980; Stefanova et al., 2002), resulting in improved signal detection at cell division sites.
Materials and Reagents
- Sterile Petri dishes 92 × 16 mm with cams (SARSTEDT, catalog number: 82.1473)
- Pipette tips 10 µl, 200 µl and 1,000 µl (STARLAB, catalog numbers: S1111-3700, S1113-1700, S1111-6701)
- 1.5 ml micro-tubes (SARSTEDT, catalog number: 72.690.001)
- Sterile serological pipettes (5, 10 and 25 ml) (SARSTEDT, catalog numbers: 86.1253.001, 86.1254.001 and 86.1685.001)
- Sterile 50 ml CELLSTAR tubes with blue screw cap (Greiner BIO-ONE, catalog number: 227261)
- Concentric Luer-Lok Syringe 50 ml (BD Plastipak, BD Luer Lok, catalog number: 10636531)
- 0.22 µm syringe filter (PES membrane, sterile) (STARLAB, catalog number: E4780-1226)
- Diagnostic microscopic slides (12 wells, 5.2 mm numbered) (Thermo Scientific, catalog number: 10028210)
- Microscope slides SuperFrost plus (25 × 75 × 1 mm) (R. Langenbrinck Labor + Medizintechnik, catalog number: 03-0060)
- Cover glasses, square (22 × 22 mm, thickness 1.5) (VWR, catalog number: 631-0125)
- Semi-micro cuvettes (polystyrene, 10 × 4 × 45 mm) (SARSTEDT, catalog number: 67.742)
- Milli-Q quality water (ddH2O) and distilled water
- Peptone from casein, enzymatic digest (Sigma-Aldrich, catalog number: 82303)
- Peptone from soybean, enzymatic digest (Fluka Analytical, catalog number: 90765)
- Sodium chloride (NaCl) (VWR, catalog number: 27810.295)
- Di-Potassium hydrogen phosphate (K2HPO4) (VWR, catalog number: 26931.263)
- D-Glucose (anhydrous) (Melford, catalog number: G1400)
- Bacto Agar (BD Diagnostics, catalog number: 214030)
- Bacto Tryptone (BD Diagnostics, catalog number: 211699)
- Yeast extract powder (Ohly KAT, catalog number: FllOHLYKAT)
- FDAA 7-hydroxycoumarincarbonylamino-D-alanine (HADA) provided by Michael S. VanNieuwenhze, Indiana University (Kuru et al., 2015)
- Dimethylsulfoxide anhydrous (DMSO) (Invitrogen, catalog number: D12345)
- Citric acid (anhydrous) (Sigma-Aldrich, catalog number: C2404)
- Sodium hydroxide (NaOH) (VWR, catalog number: 28245.298)
- 10× PBS buffer with 0.017 M KH2PO4, 0.05 M Na2HPO4, 1.5 M NaCl, pH 7.4 (phosphate saline) (Lonza Walkersville INC, AccuGENE, catalog number: 51226)
- 16% Paraformaldehyde (formaldehyde) aqueous solution (Electron Microscopy Science, catalog number: 15710-S)
- SB Molecular Biology Grade Agarose (Severn Biotech LtD., catalog number: 30 10 50)
- Tryptic soy broth (TSB) and Tryptic soy agar (TSA) (see Recipes)
- Miller Luria-Bertani (LB) medium and LB agar (see Recipes)
- HADA stock solution (see Recipes)
- 10× Sodium citrate buffer, pH 2.25 (see Recipes)
- 1× sodium citrate buffer, pH 3.0 (see Recipes)
- 1× Phosphate buffered saline pH 7.4 (PBS) (see Recipes)
- 3% Paraformaldehyde solution (see Recipes)
- Agar slides for microscopic imaging (see Recipes)
Equipment
- Sterile, flexible and disposable Inoculating loops (VWR, catalog number: 612935P)
- Glass beaker, 600 ml and 1,000 ml (PYREX, catalog numbers: 1000/18D and 1000/22D, respectively)
- Premium single Cell Analytical Balance (Kern, model: ABT 120-4NM)
- IKA RH basic 2 magnetic stirrer (IKA, catalog number: 0003339002)
- pH meter (Cole-Parmer, Jenway, model: 3510, catalog number: 351001)
- Pipettes (Gilson, catalog numbers: F167300 and F167500)
- Ultra Low Temperature Freezer VIPTM Series -86 °C (SANYO, catalog number: MDF-U73V)
- Comfort TP 1410 table-high, under worktop fridge (Liebherr, catalog number: 4016803034339)
- Vortex (IKA, model: Minishaker MS2)
- Microwave MS106 0.6 FT (Matsui)
- 50 L Laboratory Incubator with Digital Control (GenLab incubator: INC/50/DIG)
- Microcentrifuge accuSpin Micro 17R, refrigerated (Fisher Scientific, catalog number: 11526873)
- Nikon Eclipse Ti microscope (Nikon Plan Fluor × 100/1.30 Oil Ph3 DLL objective) equipped with a Photometrics/Cool SNAP HQ2CCD camera using the phase contrast and DAPI channel (filter set: Chroma 49000, excitation at 350/50 nm, emission 460/50 nm)
- Autoclave
Software
- ImageJ (Rasband W.S./U. S. NIH, Bethesda, Maryland, USA/https://imagej.nih.gov/ij/)
- MetaMorph® Version 7.0 (Microscopy Automation and Image Analysis Software/Molecular DevicesLLC/https://www.moleculardevices.com/products/cellular-imaging-systems/acquisition-and-analysis-software/metamorph-microscopy)
Procedure
文章信息
版权信息
© 2019 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Peters, K., Pazos, M., VanNieuwenhze, M. S. and Vollmer, W. (2019). Optimized Protocol for the Incorporation of FDAA (HADA Labeling) for in situ Labeling of Peptidoglycan . Bio-protocol 9(15): e3316. DOI: 10.21769/BioProtoc.3316.
分类
微生物学 > 微生物细胞生物学 > 细胞染色
细胞生物学 > 细胞染色 > 细胞壁
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link