- EN - English
- CN - 中文
Optical Stimulation and Electrophysiological Analysis of Regenerating Peripheral Axons
光刺激和电生理学分析再生外周轴突
发布: 2019年06月20日第9卷第12期 DOI: 10.21769/BioProtoc.3281 浏览次数: 5637
评审: Shaarika SarasijaAnonymous reviewer(s)
Abstract
Although axons in the peripheral nervous system can regenerate, functional recovery after nerve injuries is poor. Activity-based therapies, such as exercise and electrical stimulation, enhance the regeneration of cut peripheral axons. Despite their effectiveness, clinical application of these experimental techniques has been limited. At least part of the basis for this translational barrier has been a lack of information as to the precise mechanism of activity-based therapies on peripheral axon regeneration. To evaluate the requirements for neuron-type specific activation to promote regeneration using these therapies, in the current protocol, we employed optogenetics. Utilizing the advantages of transgenic mouse lines we targeted opsin expression to different neuron types. Using fiber optics we activated those neurons with high temporal specificity as a model of activity-based intervention after nerve injury and to measure functional recovery achieved after such a treatment.
Keywords: Channelrhodopsin (光敏感通道)Background
Optogenetics has emerged as a potent tool in experimental neuroscience. The expression of light-gated ion channels in neurons can be used to control neuronal activity with great temporal precision, and by targeting of that expression to different neuronal populations the role of that activity can be studied very specifically. Our lab and others have demonstrated that activity-based experimental therapies, such as exercise and electrical stimulation, if applied after peripheral nerve injury will enhance the regeneration of cut axons of motor and sensory neurons (Al-Majed et al., 2000; Brushart et al., 2002; Wood et al., 2012; Thompson et al., 2014). Exercise in the form of treadmill running is a powerful promoter of axon regeneration but it is difficult to separate the effects of exercise-induced activation of injured neurons from other effects, such as alterations in available hormones, changes in muscle properties, modulation of immune cells or activation of uninjured neurons in networks that generate the locomotion. Furthermore, when modeling the effects of exercise on recovery from peripheral nerve injury, it is difficult to determine whether the effect is due to the production of action potentials in the neurons whose axons have been severed or whether exercise may alter the membrane potential of axotomized neurons without directly causing it to fire an action potential. To determine these parameters would be highly challenging in an awake behaving animal running on a treadmill.
Brief electrical stimulation of a cut peripheral nerve also enhances axon regeneration (Elzinga et al., 2015; Gordon and English, 2016). It offers some technical advantages in probing the mechanism of action of exercise on peripheral axon regeneration. A specific peripheral nerve can be unilaterally targeted by applying stimulation to the nerve, and the frequency and duration of stimuli (action potentials) applied to the nerve can be controlled. However, other cell types within a peripheral nerve also respond to electrical stimulation (Schwann cells and probably immune cells), and the conductive nature of tissues can facilitate current spread. The lack of cell-type specificity of electrical stimulation and exercise also have limited some of the mechanistic questions that can be proposed. Here, we describe a way to use optogenetics to apply a cell-type specific activity-based therapy and also to use optogenetics to provide a functional outcome measure (direct muscle response, i.e., the direct M response) following nerve injury.
We used mouse genetics to target the expression of the light-sensitive cation channel, channelrhodopsin 2, selectively in sensory neurons, motoneurons, or both. In the mice we used, only a subset of the neurons with axons in peripheral nerves expressed this opsin, those that also express yellow fluorescent protein (YFP); other neurons acted as internal controls. We then activated the opsin-expressing neurons by application of light to their axons in peripheral nerves. We found that brief optogenetic activation enhanced peripheral axon regeneration regardless of whether motor or sensory neurons were treated individually or synchronously (Figure 1). We also used optogenetics to measure functional recovery using electrophysiology (Ward et al., 2016).
A major advantage of the use of optogenetics in this model system is that activity can be discretely controlled in genetically targeted neuronal populations of interest. Optogenetics can be used to evaluate the mechanism behind the success of activity-based therapies on peripheral axon regeneration. For example, we have also used an inhibitory opsin, Natromonas halorhodopsin, fused to a light-emitting Renilla luciferase and Venus (a protein for bioluminescent resonance energy transfer that amplifies the emitted light) (Tung et al., 2015; Berglund et al., 2016) to inhibit neuronal activity during treadmill exercise. The luciferase oxidizes a small molecule substrate, h-Coelenterazine, to generate yellow photons and activate the halorhodopsin. Thus, after administration of h-Coelenterazine, neurons expressing this iLMO2 fusion protein become hyperpolarized. In motoneurons whose axons were regenerating, we found that inhibiting motoneuron activity during treadmill exercise blocked the enhancing effect of exercise (Jaiswal et al., 2017). Considered together, the results of these optogenetic experiments lead us to conclude that neuronal activity is both necessary and sufficient to promote the regeneration of injured peripheral axons.
Future advances in excitatory and inhibitory opsins, bioluminescence, luminopsins and chemogenetics will continue to facilitate the development of neuromodulation and novel gene therapy approaches. Via chemogenetic manipulation using DREADDs (Cre-dependent excitatory designer receptor exclusively activated by designer drugs), we have shown that subthreshold excitation of motoneurons and sensory neurons is sufficient to enhance their regeneration (Jaiswal et al., 2018). The application of these new optogenetic tools will expand our ability to further define the activity requirements of highly specific neuronal populations.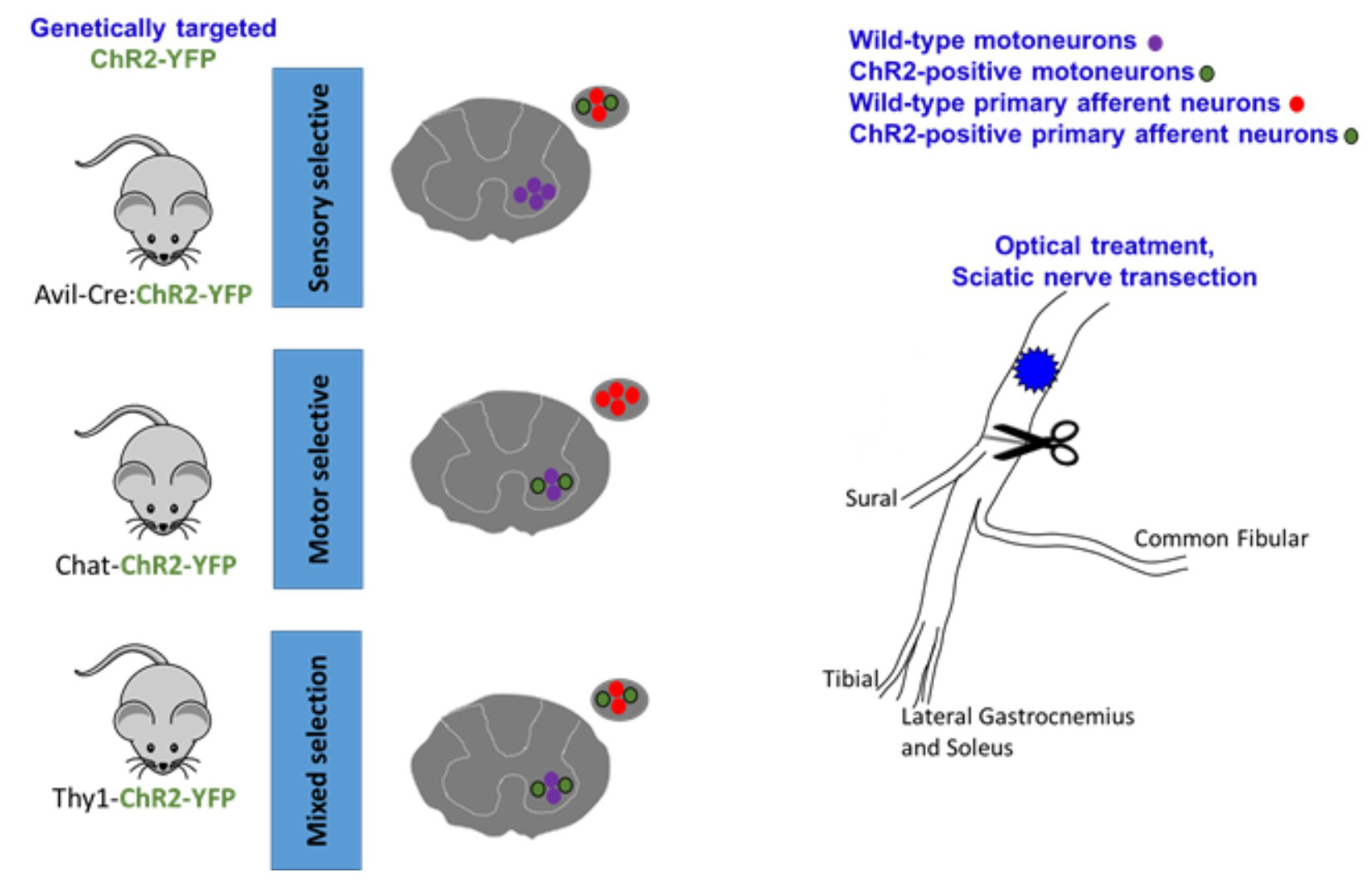
Figure 1. graphical representation of the mouse model and sciatic nerve. Multiple promoters can be used to selectively express ChR2 (or other opsins) in targeted cell types. In the cartoons of spinal cords and dorsal root ganglia cross-sections, different cell types express ChR2 and are responsive to blue light activation in the periphery. The whole sciatic nerve and its terminal branches are depicted in the cartoon. Using different transgenic mouse models, we have shown that excitatory neuronal stimulation and exercise enhance axon regeneration following sciatic nerve injury, and this effect can be blocked with inhibitory luminopsins.
Materials and Reagents
- 0.5 ml tubes (Bio PLAS, Inc.)
- Silastic sheeting (Dow Corning Corporation Medical Products)
- 10 μl pipette tips (BioExpress)
- 1 ml Syringe 25 G (BD, Franklin lakes, NJ)
- Gauze pads 2 x 2 (Fisher brand)
- Betadine wipes (Purdue Products, L.P.)
- Coated Visorb undyed braided polyglycolic acid suture NSF-2 19 mm 3/8 30” (75 cm)
- Monofilament polyamide suture (Redilon 5-0) (1 metric) 18” (45 cm)
- Mice expressing desired opsins (Jackson Laboratories)
- Thrombin (MP Biomedical)
- Fibrinogen (Sigma)
- Xylazine sterile solution 20 mg/ml Ana Sed Injection (AKORN)
- Ketamine HCl Injection, USP 50 mg/ml (Hospira)
- Ethanol (Fisher)
- CaCl2 (Sigma)
- Fibrin glue (see Recipes)
Equipment
- Surgery
- Dumont forceps #5 (Fine Science Tools, catalog number: 11293-00)
- Spring scissors (Fine Science Tools, catalog number: 15008-08)
- Surgical scissors (Fine Science Tools, catalog number: 14084-09)
- Suture tying forceps (Fine Science Tools, catalog number: 11090-09)
- Needle holders with suture cutters (Fine Science Tools, catalog number: 12502-14)
- Hair clipper (Braintree Scientific)
- Heating pad or warm water-circulator (Braintree Scientific Gaymar TP500)
- Scalpel and handle
- Surgical microscope (Wild Heerbrug Stereozoom)
- Table clamp (to hold the fiber optic cable in place)
- 2.5-10 μl pipette
- Glass-bead sterilizer (Braintree Scientific, catalog numbers: GER 5287-120V and GER 5289)
- -20 °C freezer
- optogenetics and electrophysiology
- Optogenetics patch cable (ThorLabs, catalog number: M82L01)
- Fiber coupled laser source and driver (ThorLabs)
Notes:- Alternatively: We built our own laser diode source and driver and have published the hardware setup in detail with photos at http://web.stanford.edu/group/dlab/optogenetics/hardware.html.
- It is important that the light source must emit light in the wavelength of the opsin being used. For example, we used blue light (473 nM wavelength) to activate channelrhodopsin 2.
- Cuff electrode (or other electrophysiologic recording devices, such as a microchannel nerve interface)
- Silastic tubing (Laboratory Tubing, catalog number: 508-006)
- insulated wire (CoonerWire, catalog number: AS631)
- flexible silicone (Dow Corning, catalog number: Sylgard® 184)
- Recording electrodes
- Fine wire, size 0.002 mm (California Fine Wire Company, MO#M468240)
- Sterile 25 G needle
- Laser safety glasses (ThorLabs, Inc.)
- Silver ground wire (A-M Systems, Inc.)
- Electrical stimulator and stimulus isolation units:
- low-noise 6-channel electrophysiology amplifiers (Figure 2A)
- optically isolated pre-amplifier stage (Figure 2B)
- Linear photovoltaic optocouplers (IXYS Integrated Circuits, catalog number: LOC111)
- Galvanically isolated power supply module (CUI, catalog number: VWRAS2-D5-D12)
- Preamp instrumentation amplifier (Analog Devices, catalog number: AD8421)
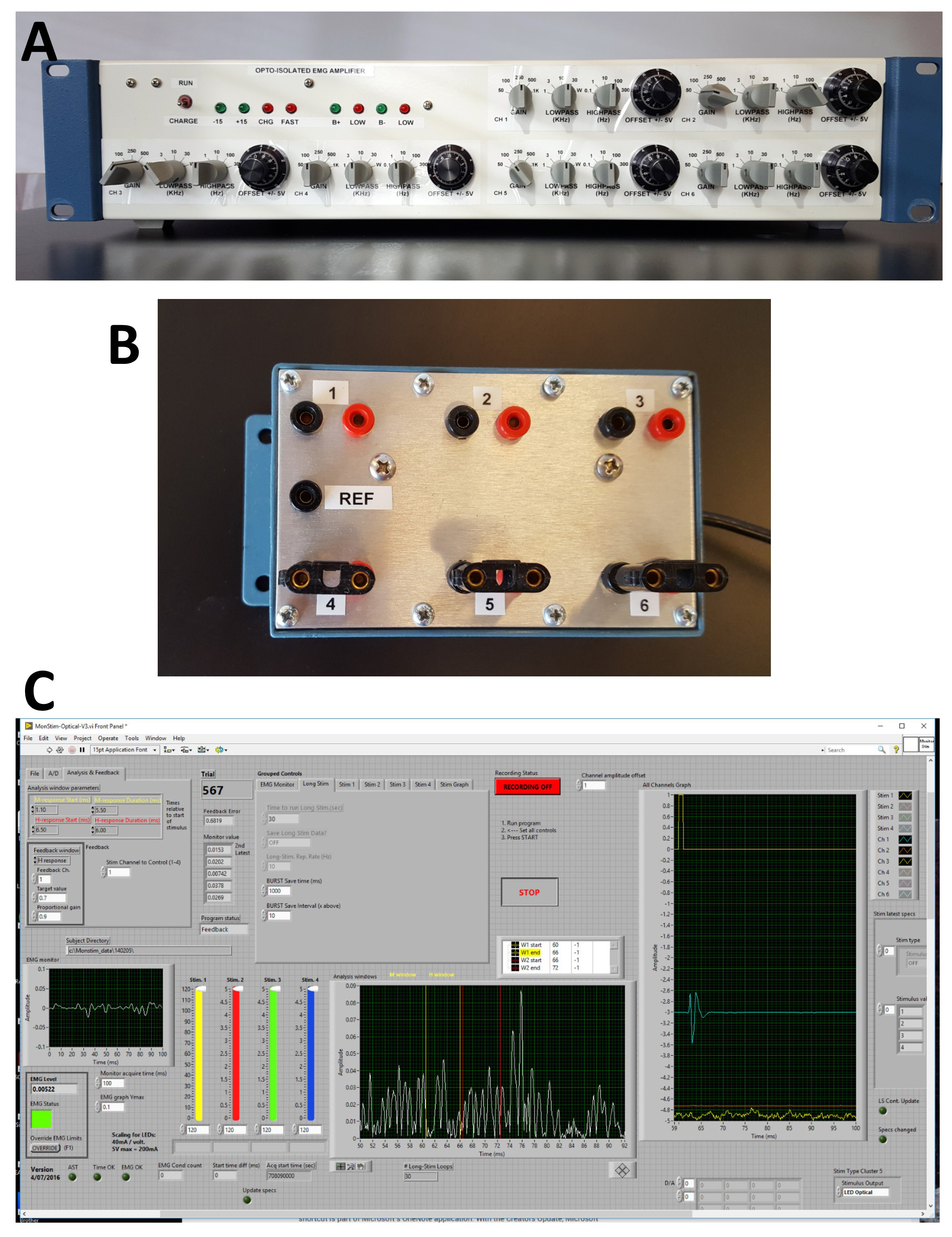
Figure 2. Electrophysiology rig and software. A. Front panel of six channel AC amplifiers used. Separate controls of gain, low pass and high pass filters and offset are present for each channel. B. The optically isolated head stage amplifier unit enables bipolar inputs for each channel and a common reference. C. Front panel of custom Labview® software used for acquisition of electrophysiological data.
Software
- Labview® (National Instruments)
Note: Other software is commercially available to run optogenetic hardware (e.g., ThorLabs). - Data acquisition and stimulation program
Our data acquisition and stimulation program were developed in-house using National Instruments’ Labview® programming language. The program monitors ongoing activity for one of the input channels for nerve or electromyography/electroneurogram (EMG/ENG) activity. This is displayed on the left of the front panel (Figure 2C). When the average value of activity falls within a user-defined voltage window for 20 ms, a stimulus is output to wire cuff electrodes or optical devices, either LEDs or laser pulses, and data are recorded for a given period, written to disc and displayed in windows to the right of the front panel (Figure 2C). The data can then be analyzed off-line as described below.
Procedure
文章信息
版权信息
© 2019 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Ward, P. J. and English, A. (2019). Optical Stimulation and Electrophysiological Analysis of Regenerating Peripheral Axons . Bio-protocol 9(12): e3281. DOI: 10.21769/BioProtoc.3281.
分类
神经科学 > 周围神经系统 > 坐骨神经
神经科学 > 感觉和运动系统 > 脊髓
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link













