- EN - English
- CN - 中文
Probing Conformational States of a Target Protein in Escherichia coli Cells by in vivo Cysteine Cross-linking Coupled with Proteolytic Gel Analysis
利用体内半胱氨酸交联结合蛋白水解凝胶分析检测大肠杆菌细胞中目的蛋白的构象状态
发布: 2019年06月20日第9卷第12期 DOI: 10.21769/BioProtoc.3271 浏览次数: 4469
评审: Manjula MummadisettiChristopher HineAnna Vangone
Abstract
Transporters are dynamic membrane proteins that are essential to the physiology of cells. To function, transporters must cycle between various conformational states, so to understand their mechanistic details, it is critical to characterize how their structure changes during the transport cycle. One approach to studying the dynamics of transporters takes advantage of the chemistry of cysteine by using sulfhydryl-reactive, bi-functional cross-linkers to probe changes in the distance between two specific residues that have been substituted to cysteine. This approach is mostly used to study transporters in vitro, not in their natural cellular environment. Here we describe a protocol based on structure-guided cysteine cross-linking and proteolysis-coupled gel analysis to probe conformational changes of a target transporter in live Escherichia coli cells. Although cross-linking approaches have been used to probe the proximity between transmembrane segments in membrane proteins in vivo, to our knowledge this protocol is the first to be used to interrogate transporter dynamics in cells. The use of this protocol is optimal for proteins with known or modeled structures to guide the replacement of specific residues with cysteines and the selection of cross-linking agents with various spacer arm lengths. This protocol allows for discriminating easily cross-linked and uncross-linked species and does not require the often difficult or unavailable reconstitution of transport activity in an in vitro system. In addition, this protocol could be used to probe the conformation of transporters in cells treated with transport inhibitors in order to better understand their mechanism of action, and potentially dynamic interactions between domains in proteins that are not transporters.
Keywords: Conformational dynamics (构象动态)Background
Transport of substrates across membranes is fundamental to all organisms. Membrane proteins that function as transporters constitute a very large and structurally diverse group of proteins that translocate a vast landscape of substrates through different mechanisms. Not surprisingly, approximately 25% of polypeptides encoded in fully sequenced genomes are predicted to be membrane proteins, and many of them are likely transporters (Elofsson and von Heijne, 2007). Their essential role in human health is underscored by the fact that genetic defects in specific transporters cause disease (e.g., cystic fibrosis), and that some transporters are targeted by pharmaceuticals (e.g., inhibitors of efflux pumps) (Meng et al., 2018; Wiese and Stefan, 2019). Studying transporters is therefore important, but it is also challenging given their dynamic nature and the inherent difficulty of working with membrane proteins in biochemical and structural studies. Especially difficult is obtaining high-resolution structures, particularly those representing different states in the transport cycle. Even when available, static structures must be validated and their role in the transport cycle elucidated by complementary in vitro and in vivo assays that might not be available for many transporters (Mulligan and Mindell, 2017).
Chemical biology approaches such as cysteine cross-linking can provide mechanistic details about transporters even in the absence of high-resolution structures (Mulligan and Mindell, 2017). Indeed, cysteine cross-linking has been used to probe the structure and conformational dynamics of many membrane proteins in vitro (Sun et al., 1997; Wu et al., 1999; Rimon et al., 2002; Hennon and Dalbey, 2014; Radchenko et al., 2016; Mulligan and Mindell, 2017). However, a concern when using this approach is that the in vitro system might not properly reflect the native environment of the target protein. The in vitro approach also requires the purification of multiple protein variants, which can be difficult for many transporters. More importantly, in vitro cysteine cross-linking relies on the functional reconstitution of the activity of purified membrane proteins, which can be extremely difficult or unavailable for many transporters. For example, developing an in vitro functional assay can be difficult for glycolipid transporters because their complex substrates are often not available. In our case, we study the bacterial transporter MurJ, which translocates the glycolipid lipid II from the inner leaflet to the outer leaflet of the cytoplasmic membrane, and for which we do not currently have an in vitro reconstitution assay to probe its function (Ruiz, 2008; Butler et al., 2013 and 2014; Sham et al., 2014; Ruiz, 2015 and 2016; Rubino et al., 2018; Kumar et al., 2019). As a result, we developed an in vivo cysteine cross-linking protocol that captures the different conformations the MurJ transporter adopts in growing Escherichia coli cells, when MurJ activity is required for viability (Kumar et al., 2019).
We designed this protocol to probe whether the V-shaped target transporter MurJ can adopt two states during the transport cycle in live bacteria: one in which the internal cavity is open to the cytoplasm (i.e., inward-open conformation) and another in which the cavity is open to the periplasm (i.e., outward-open conformation). Based on a crystal structure assumed to reflect a single state in the transport cycle, these conformations were predicted to result from the relative movement of two large domains or lobes that traverse the membrane and give the transporter its V shape (Kuk et al., 2017; Zheng et al., 2018). To test this model and obtain evidence that these states exist in cells, we first introduced a cysteine residue into either the periplasmic (out) or the cytoplasmic (in) side of each of the lobes (N-lobe and C-lobe) of our otherwise cysteine-less transporter. We then used homo-bifunctional cross-linking reagents of various lengths that could cross-link specific cysteine pairs when the two lobes adopted only one of the two predicted states, inward-open (Figure 1A) or outward-open (Figure 1B). To easily distinguish cross-linked and uncross-linked target proteins, we engineered a functional variant of the target protein containing a well-known protease site (thrombin) in the loop connecting the N and C lobes that could be cleaved after cells were treated with cross-linkers (Figure 2A). In addition to the protease site, a fusion tag (FLAG) was also added to the N terminus of the target protein (Figure 2A) for detection by immunoblotting (Figure 2B). Using this protocol, we demonstrated that, in vivo, the glycolipid MurJ transporter adopts both inward- and out-ward open conformations and hence uses the alternate-access mechanism. Furthermore, we also showed that the transporter stalls in the outward-open state in cells treated with ionophores that collapse the membrane potential (Kumar et al., 2019). We therefore showed that our protocol can be used 1) to test the mechanism of transport by monitoring the distance between specific sites in a transporter during the transport cycle in live cells, 2) to validate high-resolution structures and in silico structural predictions, and 3) to test the effects of small-molecule inhibitors of transporters. It should also be noted that our protocol could also be used to probe dynamic interactions between domains of proteins that are not transporters (Kumar et al., 2019).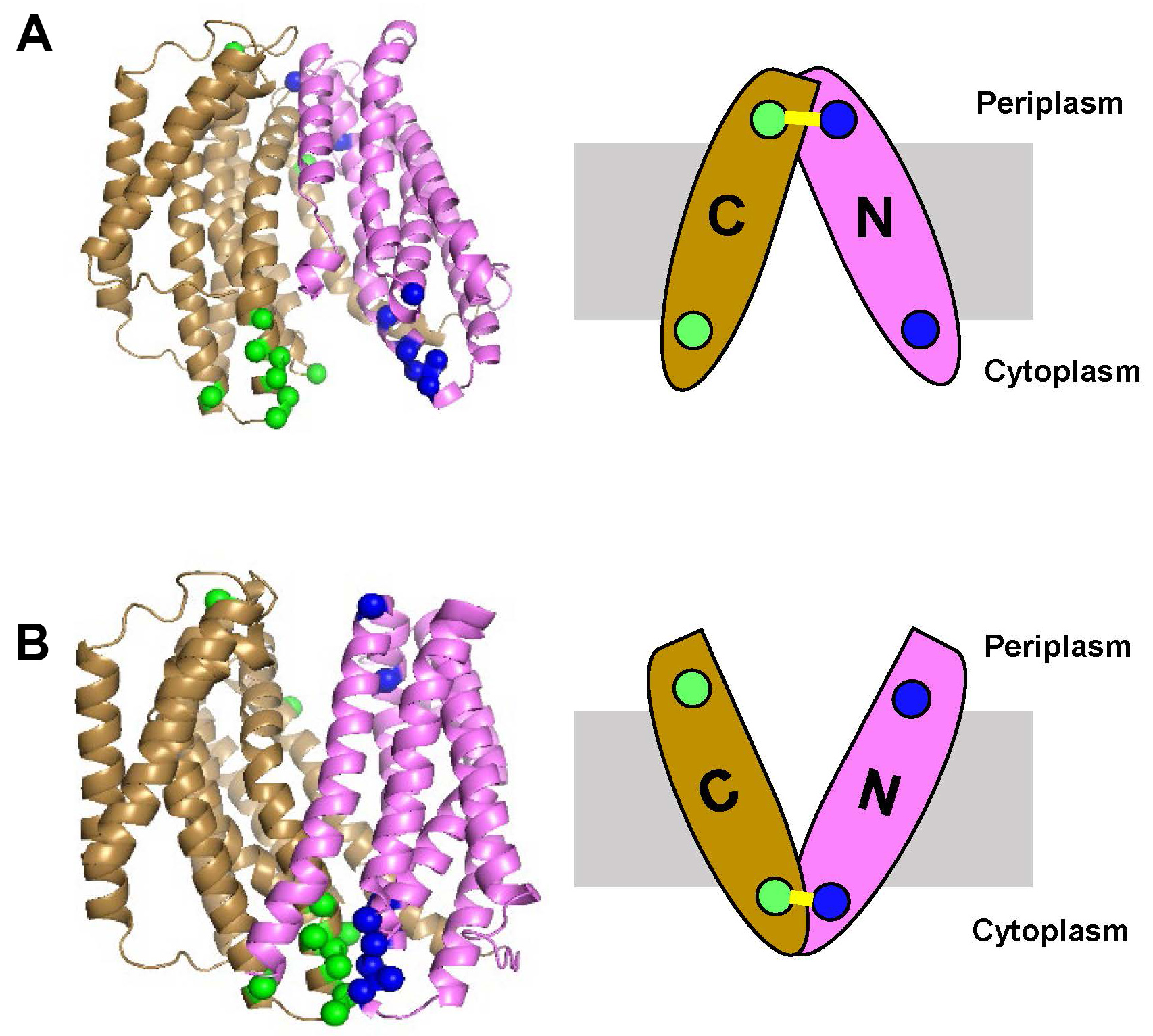
Figure 1. Selection of residues on target protein (E. coli MurJ) to be modified to cysteines for in vivo cross-linking. On the left, side view of the inward-open structure (panel A, Protein Data Bank ID code 6CC4) and outward-open homology model of MurJ (panel B). The N lobe (TM1-6) is colored pink and C lobe (TM7-14) is colored gold. The positions of amino acids to be substituted to cysteines in both periplasmic and cytoplasmic sides are denoted as blue spheres for those in the N lobe and green spheres for those in the C lobe. On the right, a cartoon representation of the structures showing that crosslinking (yellow line) can only occur in one conformation: between periplasmic cysteines closed enough to crosslink in the inward-open conformation (panel A) or between cytoplasmic cysteines closed enough to crosslink in the outward-open conformation (panel B). The membrane is represented with a grey box. PyMOL (version 2.0.1, Schrodinger) software was used to prepare this figure.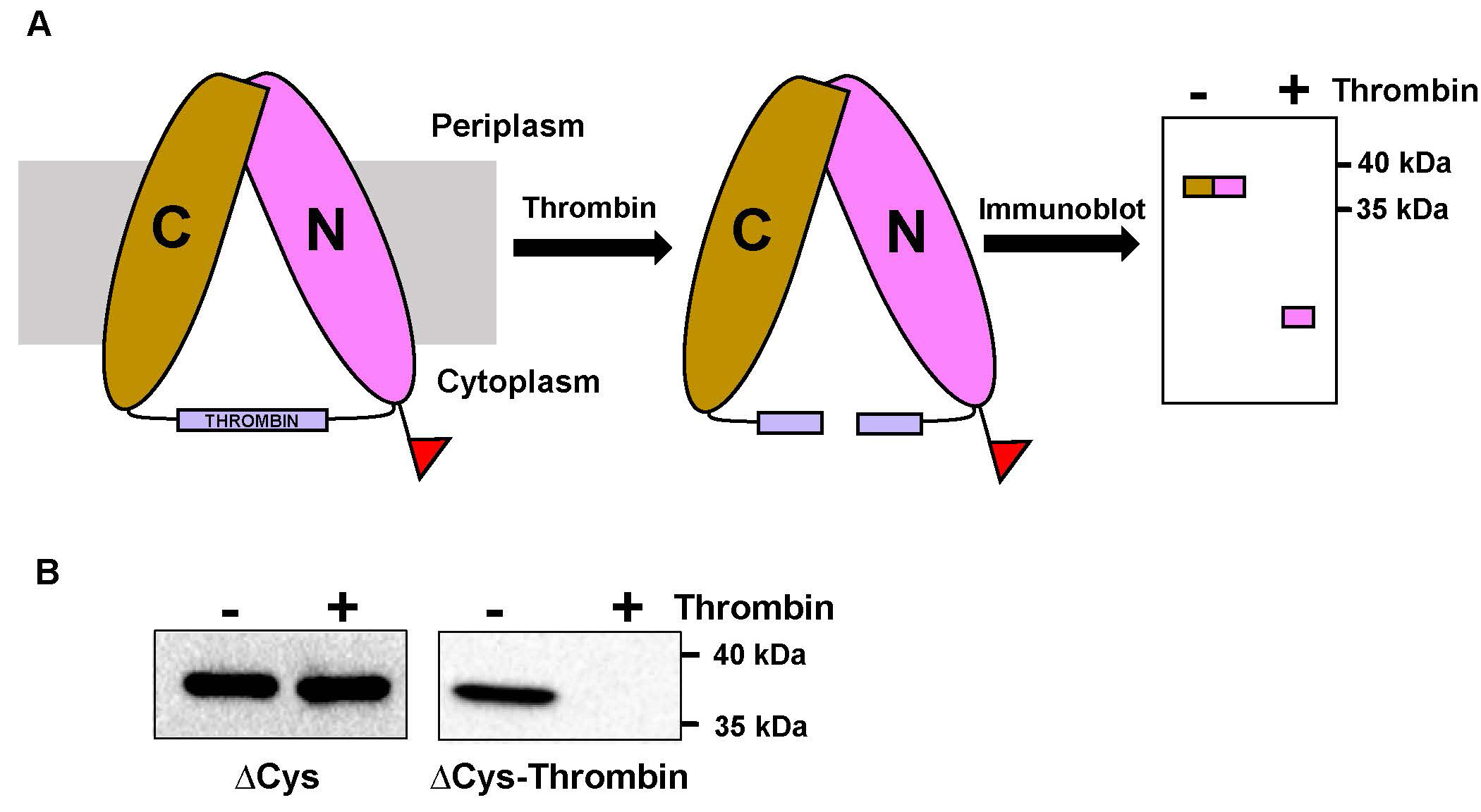
Figure 2. Proteolytic analysis of target protein variant utilized for probing the conformational states in vivo. A. Cartoon representation of the target protein (E. coli MurJ) variant and its proteolytic analysis. A thrombin protease site (purple box) was introduced between the N lobe (pink) and C lobe (gold) of cysteine-less target protein (∆Cys). An N-terminal FLAG tag used for detection by immunoblotting is shown as a red flag on the N lobe. In the absence of thrombin treatment, the full-length ∆Cys-thrombin variant should appear as a ~37 kDa band in a FLAG immunoblot. Thrombin treatment should cleave the two lobes, so only a fast-migrating band corresponding to the N lobe should be detected by FLAG immunoblotting. B. Result of thrombin treatment of full-length FLAG-MurJ∆Cys (∆Cys) and FLAG-MurJ∆Cys-thrombin (∆Cys-Thrombin) proteins. The small fragment resulting from thrombin digestion is not shown as it runs off the 10% SDS-polyacrylamide gel used for immunoblotting. We used this type of gel because it is optimal for the detection of the full-length target protein.
Materials and Reagents
- Nunc cryotube vials (Thermo Scientific, catalog number: 374502)
- Bacteriological Petri plates (VWR, catalog number: 25384-092)
- Sterile test tubes (17 x 100 mm, RPI, Research Products International, catalog number: 168599)
- 15-ml centrifuge tubes (VWR, catalog number: 10025-690)
- 50-ml centrifuge tubes (GeneMate, catalog number: C-33943-3)
- 1.7-ml microcentrifuge tubes (Axygen, catalog number: MCT-175-C)
- Ultracentrifuge tubes (Beckman Coulter, catalog number: 343778)
- Serological pipettes: 5-ml pipette (VWR, catalog number: 89130-896), 10-ml pipette (VWR, catalog number: 89130-910), 25-ml pipette (VWR, catalog number: 89130-910)
- 0.1-10 μl pipette tips (GeneMate, catalog number: P-1236-10)
- 20-200 μl pipette tips (GeneMate, catalog number: P-1236-200)
- 101-1,000 μl pipette tips (GeneMate, catalog number: P-1236-1250)
- 0.2 μm filter (Millipore, Model; MillexGP, catalog number: 202012)
- Aluminum foil
- All-purpose wrap (Fisher Brand, catalog number: 15-610)
- Nitrile or latex gloves (VWR, catalog number: 89038-270)
- Microscope slides (Corning, catalog number: 2947-75X25)
- Thick Blot paper (Bio-Rad, catalog number: 1703965)
- Escherichia coli crosslinking strains carrying derivatives of plasmid pET23/42FLAGMurJ∆cysThrombin (Dr. Natividad Ruiz strain database) (Kumar et al., 2019)
Note: These strains will vary depending on the target protein and plasmids available. - Immun-Blot PVDF membrane (Bio-Rad, catalog number: 1620177)
- Trans-Blot SD semidry transfer cell (Bio-Rad, catalog number 1703848)
- Ice
- Distilled sterile water (dH2O)
- LB Broth, Lennox (Fisher Scientific, catalog number: BP1427-2)
- Difco-Agar Bacteriological (BD, catalog number: BD-214510)
- Ampicillin sodium salt (Sigma-Aldrich, catalog number: A9518-5G) or other antibiotic appropriate for the selection of plasmids encoding other target proteins
- 1,6-bis(maleimido)hexane (BMH; Thermo Scientific Pierce, catalog number: 22330)
- 1,2-bis(maleimido)ethane (BMOE; Thermo Scientific Pierce, catalog number: 22323)
- o-PDM (Sigma-Aldrich, catalog number: 104590-5G)
- L-cysteine (Sigma-Aldrich, catalog number: 168149-5G)
- Lysozyme (Amresco, catalog number: 0663-5G)
- Benzonase (Sigma-Aldrich, catalog number: E1014 5KU)
- Thrombin from bovine plasma (Sigma-Aldrich, catalog number: T6634-100UN)
- Ethylenediaminetetraacetic acid disodium dihydrate (EDTA) (Sigma-Aldrich, catalog number: E5134-1KG)
- Calcium chloride (CaCl2) (Sigma-Aldrich, catalog number: 223506-500G)
- Ammonium persulfate (Amresco, catalog number: 0486-100G)
- N-Dodecyl-β-D-maltoside (DDM; Affymetrix, catalog number: D310S)
- Magnesium chloride (MgCl2) (Sigma-Aldrich, catalog number: T6634-100UN)
- Sodium citrate tribasic dihydrate (Sigma-Aldrich, catalog number: C8532-1KG)
- Disodium hydrogen phosphate heptahydrate (Na2HPO4·7H2O) (Amresco, catalog number: 0348-1KG)
- Sodium dihydrogen phosphate (NaH2PO4) (Fischer Scientific, catalog number: S397-500)
- Sodium chloride (NaCl) (Sigma-Aldrich, catalog number: 59888-2.5KG)
- PEG-8000 (Sigma-Aldrich, catalog number: 89510-250G)
- Sucrose (Amresco, catalog number: 0335-1KG)
- Dimethyl sulfoxide (DMSO) (Amresco, catalog number: 0231-500ml)
- Tris (GoldBioCom, catalog number: T-4005-5)
- Sodium dodecyl sulfate (SDS) (Sigma-Aldrich, catalog number: 62862-1KG)
- Urea (Ameresco, catalog number: 0568-1KG)
- Bromophenol blue sodium (Amresco, catalog number: 0312-50G)
- Glycerol (Sigma, catalog number: G7893)
- β-mercaptoethanol (Amresco, catalog number: 0482-250ml)
- Acrylamide (Protogel) (National diagnostic, catalog number: EC-890)
- Glycine (Fisher Scientific, catalog number: BP381-5)
- Tetramethylethylenediamine (TEMED) (Bio-Rad, catalog number: 161-0800)
- Instant non-fat dry milk powder
- Anti-FLAG M2 (Sigma-Aldrich, catalog number: F3165)
- Methanol (Fisher, catalog number: A412P-4)
- Tween 20 (Amresco, catalog number: 0777-1L)
- ECLTM anti-mouse IgG, horseradish peroxide-linked whole antibody (from sheep) (GE healthcare, catalog number: NA931V)
- Clarity Western ECL substrate (Bio-Rad, catalog number: 1705061)
- Spheroplast buffer (see Recipes)
- Thrombin-reaction buffer (see Recipes)
- Thrombin-reconstitution buffer (see Recipes)
- Benzonase dilution buffer (see Recipes)
- 2x AB Sample buffer (see Recipes)
- Phosphate Buffered Saline (1x PBS; see Recipes)
- 10x SDS-PAGE running buffer (see Recipes)
- 1x Transfer buffer (see Recipes)
- 10x TBS Buffer (see Recipes)
- 1x TBS + 0.1% Tween 20 (1x TBST) (see Recipes)
- 5% Milk TBST (see Recipes)
- 1 M L-cysteine (see Recipes)
- Maleimide homo-bifunctional cross-linkers (see Recipes)
Equipment
- Microscope (Nikon, model: Eclipse E200)
- pH meter (Thermo scientific, model: STARA1110)
- 37 °C Incubator (Fisher Scientific, model: isotemp)
- Roller drum (Eppendorf, New BrunswickTM, model: TC-7)
- 4 °C refrigerator
- -20 °C freezer
- -80 °C freezer
- 250-ml flasks (Pyrex USA, catalog number: 4980)
- Platform shaker
- Weighing balance (Mettler Toledo, model: ML32002E/03 and AE163)
- 0.5-10 μl Pipettor (USA Scientific, model: Ergo One, catalog number: 7100-0510)
- 2-20 μl Pipettor (USA Scientific, model: Ergo One, catalog number: 7100-0220)
- 20-200 μl Pipettor (USA Scientific, model: Ergo One, catalog number: 7100-2200)
- 100-1,000 μl Pipettor (USA Scientific, model: Ergo One, catalog number: 7110-1000)
- PIPETMAN® (JENCONS, model: Powerpippette plus)
- Table-top centrifuge (Eppendorf, model: 5810R)
- High-speed microcentrifuge (Eppendorf, model: 5418)
- Clay Adams Nutator (Marshal Scientific, catalaog number: CANU)
- Ultracentrifuge (Beckman Coulter, model: OptimaMax-TL ultra)
- Ultracentrifuge fixed angle rotor (Beckman Coulter, model: TLA120.2)
- ChemiDocTM XRS+ Imaging system (Bio-Rad)
- Biowave Personal Cell Density Meter (Biochrome, catalog number: 5300-06)
- Vortex Genie 2 (Scientific Industries, catalog number: SI-0236)
- Mini-PROTEAN® Tetra Handcast Systems (Bio-Rad, catalog number: 1658004)
- PowerPac Basic power supply (Bio-Rad, catalog number: 1645050)
Software
- ImageLab (Bio-Rad)
- PyMOL (version 2.0.1, Schrodinger)
Procedure
文章信息
版权信息
© 2019 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Kumar, S. and Ruiz, N. (2019). Probing Conformational States of a Target Protein in Escherichia coli Cells by in vivo Cysteine Cross-linking Coupled with Proteolytic Gel Analysis. Bio-protocol 9(12): e3271. DOI: 10.21769/BioProtoc.3271.
- Kumar, S., Rubino, F. A., Mendoza, A. G. and Ruiz, N. (2019). The bacterial lipid II flippase MurJ functions by an alternating-access mechanism. J Biol Chem 294(3): 981-990.
分类
微生物学 > 微生物生物化学 > 蛋白质
生物化学 > 蛋白质 > 活性
生物化学 > 蛋白质 > 结构
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
提问指南
+ 问题描述
写下详细的问题描述,包括所有有助于他人回答您问题的信息(例如实验过程、条件和相关图像等)。
Share
Bluesky
X
Copy link