- EN - English
- CN - 中文
Delivering "Chromatic Bacteria" Fluorescent Protein Tags to Proteobacteria Using Conjugation
利用接合生殖将产色细菌荧光蛋白标签导入变形杆菌
发布: 2019年04月05日第9卷第7期 DOI: 10.21769/BioProtoc.3199 浏览次数: 8671
评审: Dennis J NürnbergLionel SchiavolinKirsten A. Copren
Abstract
Recently, we published a large and versatile set of plasmids, the chromatic bacteria toolbox, to deliver eight different fluorescent protein genes and four combinations of antibiotic resistance genes to Gram-negative bacteria. Fluorescent tags are important tools for single-cell microbiology, synthetic community studies, biofilm, and host-microbe interaction studies. Using conjugation helper strain E. coli S17-1 as a donor, we show how plasmid conjugation can be used to deliver broad host range plasmids, Tn5 transposons delivery plasmids, and Tn7 transposon delivery plasmids into species belonging to the Proteobacteria. To that end, donor and recipient bacteria are grown under standard growth conditions before they are mixed and incubated under non-selective conditions. Then, transconjugants or exconjugant recipients are selected on selective media. Mutant colonies are screened using a combination of tools to ensure that the desired plasmids or transposons are present and that the colonies are not containing any surviving donors. Through conjugation, a wide range of Gram-negative bacteria can be modified without prior, often time-consuming, establishment of competent cell and electroporation procedures that need to be adjusted for every individual strain. The here presented protocol is not exclusive for the delivery of Chromatic bacteria plasmids and transposons, but can also be used to deliver other mobilizable plasmids to bacterial recipients.
Keywords: Fluorescent proteins (荧光蛋白)Background
Fluorescent proteins have become an important tool for the study of bacteria, e.g., in biofilms, gene expression studies, or host-microbe interactions (Tomlin et al., 2004; Monier and Lindow, 2005; Ma and Bryers, 2010; Remus-Emsermann et al., 2011; Schada von Borzyskowski et al., 2015; Remus-Emsermann et al., 2016a; Remus-Emsermann and Schlechter, 2018; Schlechter et al., 2018).
In a recently published study, we have described the construction of 96 plasmids, each different in fluorescent protein gene, antibiotic resistance marker combination, or plasmid backbone. The complete collection offers eight different fluorescent protein genes, each with its own unique excitation and emission spectrum, four different antibiotic resistance marker combinations, a broad host range plasmid, or delivery plasmids based on two different transposon systems, Tn5 and Tn7 (Schlechter et al., 2018).
The broad-host plasmid backbone and the two transposon systems have different advantages and disadvantages: 1) The plasmid usually exists in several copies in the recipient cell, which results in multiple gene copies and thereby often higher fluorescent signals. However, plasmids may be lost more easily, leading to subpopulations of cells that might lose their ability to express fluorescent proteins (Bahl et al., 2004). 2) The Tn5 transposon inserts into Proteobacteria with high efficiency and, as it integrates into the bacterial genome, the chance of losing the fluorescent protein tag is minimal. However, the Tn5 transposon randomly inserts DNA into the genome, thereby potentially disrupting genes that are important for bacterial fitness (de Lorenzo et al., 1990). 3) The Tn7 transposon integrates into the genome of the recipient bacterium in a site-specific manner, where no genes are disrupted and thereby should have minor impact on the bacterial fitness. However, the Tn7 transposon only inserts in a narrow range of bacteria, e.g., most γ-Proteobacteria, some α-Proteobacteria, and β-Proteobacteria. Outside of the γ-Proteobacteria, its insertion frequency may be low (McKenzie and Craig, 2006; Schlechter et al., 2018).
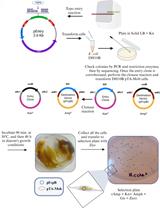
In this protocol, we describe the procedure to equip a bacterial strain with fluorescent labels using Pantoea eucalypti 299R (Remus-Emsermann et al., 2013; Tecon and Leveau, 2016) as an example recipient strain. P. eucalypti 299R is a competent recipient of all three different kinds of delivery systems, the broad host range plasmid and the two transposon systems. We could show that the broad-host plasmid as well as Tn5 and Tn7 transposon-based delivery of fluorescent proteins is feasible in this recipient strain (Schlechter et al., 2018). Other Enterobacteriaceae, such as E. coli, Erwinia amylovora and Pantoea vagans were also successfully tested with all three systems as well as Pseudomonas strains such as Pseudomonas citronellolis P3B5 (Remus-Emsermann et al., 2016b; Schlechter et al., 2018). Additionally, bacteria from other taxa, such as the α-Proteobacterial genera Sphingomonas and Methylobacterium were modified at least with the Tn5 transposon delivery system and in some cases with the broad-host-range plasmids and Tn7 transposons.
The here presented protocol is based on bacterial mating using the E. coli S17-1 helper strain, which is able to transfer mobilizable plasmids to recipients. Conjugation is a promiscuous process that crosses strain, genus, and phylum level and even works across domains of life (Heinemann and Sprague, 1989; Waters, 2001). Transformation methods such as heat-shock and electroporation are often used when transferring genetic material into laboratory model hosts, however, newly isolated strains are usually recalcitrant to these methods and the establishment of transformation protocols is often tedious and time-consuming. By relying on conjugation, transformation can be avoided and can be as simple as scraping recipient bacterial colonies from an agar plate and mixing them with the donor bacterium.
Materials and Reagents
- Autoclaved, untreated, wooden toothpicks (local supermarket of choice)
- Pipette tips, 100-1,000 μl
- Pipette tips, 1-200 μl
- Pipette tips, 0.1-10 μl
- 1.5 ml Eppendorf tubes (Corning, Axygen®, catalog number: MCT-150-C)
- 0.2 ml PCR tubes (Merck, Corning®, catalog number: CLS6531-960EA)
- Parafilm (Bemis, catalog number: PM996)
- Petri dishes 90 mm diameter (Merck, catalog number: P5731-500EA)
- Disposable cuvettes (Merck, catalog number: Z330418-1PAK)
- Disposable loops (Citotest Labware Manufacturing Co., catalog number: 2121-0001)
- Disposable spreaders (VWR, catalog number: 612-1560)
- 15 ml centrifuge tubes (Electron Microscopy Sciences, catalog number: 64760-01)
- 0.22 µm membrane filter (Merck, catalog number: GSTF02500)
- Donor strain E. coli S17-1 equipped with plasmids listed in Table 1 (strains can be acquired from Addgene, https://www.addgene.org/browse/article/28196767/)
- Recipient strain (e.g., Pantoea eucalypti 299R)
- Plasmids (Table 1) encoding for fluorescent proteins (Table 2)
Table 1. Plasmids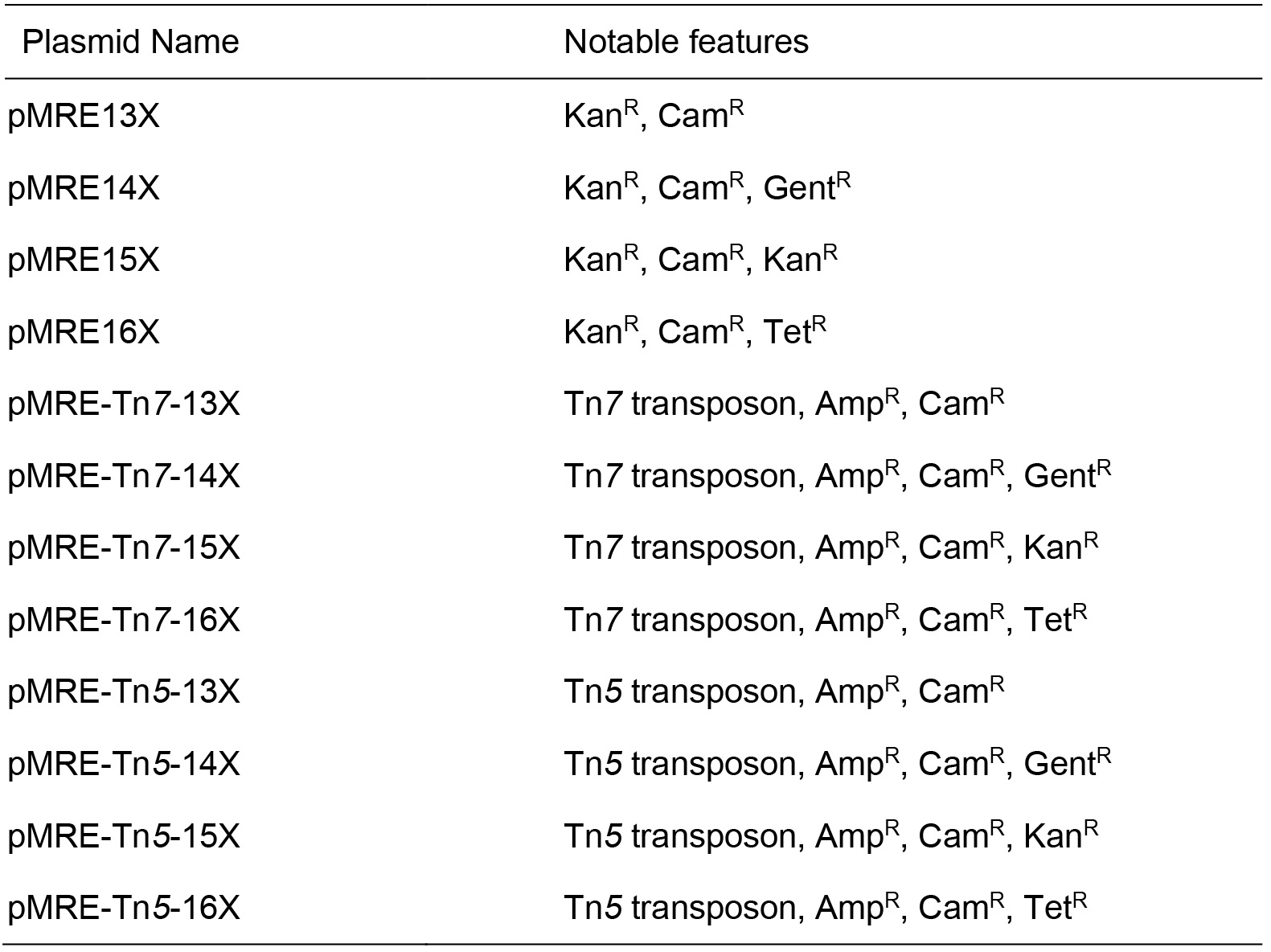
Source of all plasmids: Schlechter et al., 2018, plasmids can be obtained at Addgene plasmid number 118484 to 118579. X is a placeholder for either 0, 1, 2, 3, 4, 5, 6, 7 representing mTagBFP2, mTurquoise2, sGFP2, sYFP2, mOrange2, mScarlet-I, mCardinal, or mClover3 encoding versions of the plasmids, respectively. KanR = Kanamycin resistance; CamR = Chloramphenicol resistance; GentR = Gentamicin resistance; TetR = Tetracycline resistance; AmpR = Ampicillin resistance.
Table 2. Fluorescent protein properties*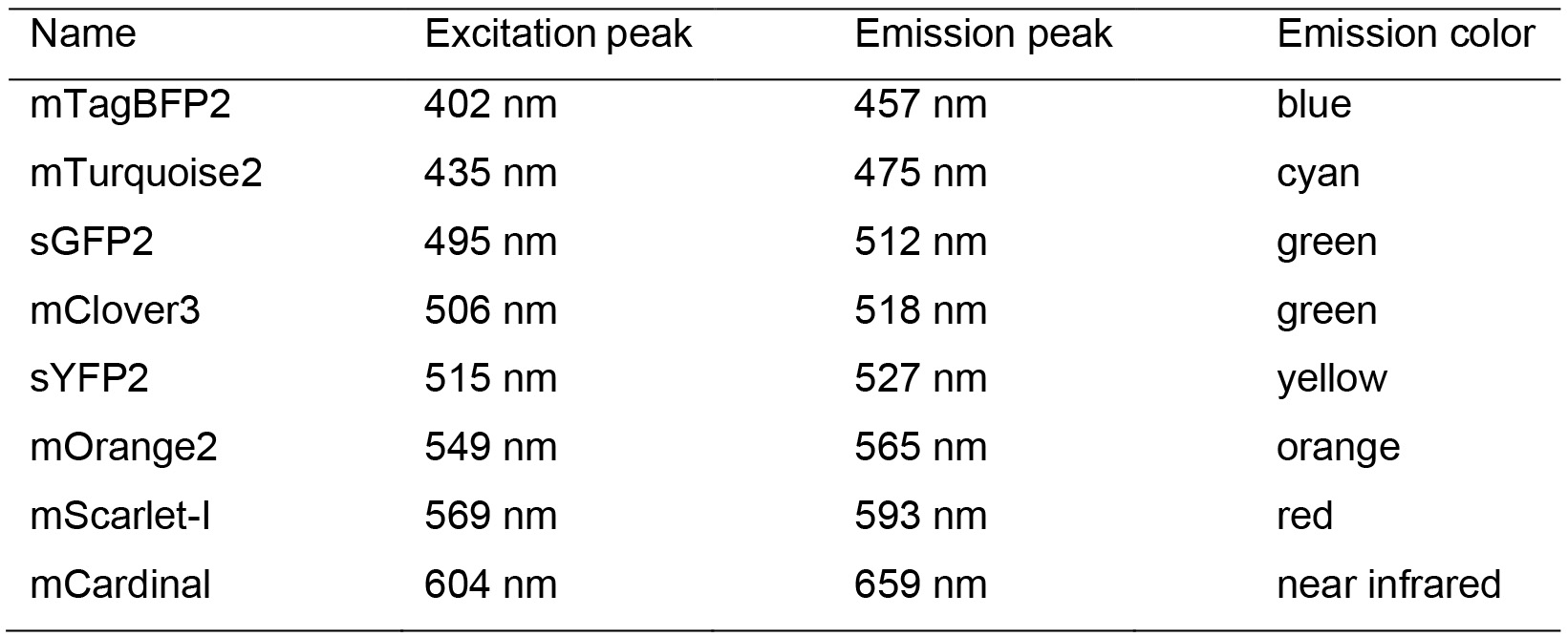
*A more detailed spectrum figure can be found in Schlechter et al., 2018 - Lysogeny broth (Merck, catalog number: 110285)
- Agar No. 1 (Oxoid, catalog number: LP0011)
- Tris free base (Sigma, catalog number: T6791-500G)
- Glacial acetic acid (Sigma, catalog number: 695092-500ML)
- Glycerol (Sigma, catalog number: G6279-500ML)
- Succinate (Sigma, catalog number: S9637-500G)
- DMSO (Sigma, catalog number: D8418-100ML)
- dNTPs (Thermo Fisher, catalog number: N8080260)
- Multi-purpose HyAgaroseTM LE Agarose (HydraGene, catalog number: R9012LE-500g)
- Phusion polymerase (Thermo Fisher, catalog number: F530S)
- Oligonucleotides, synthesis amount 0.025 µmol, purified by desalting (see Table 3, Macrogen)
Table 3. Primers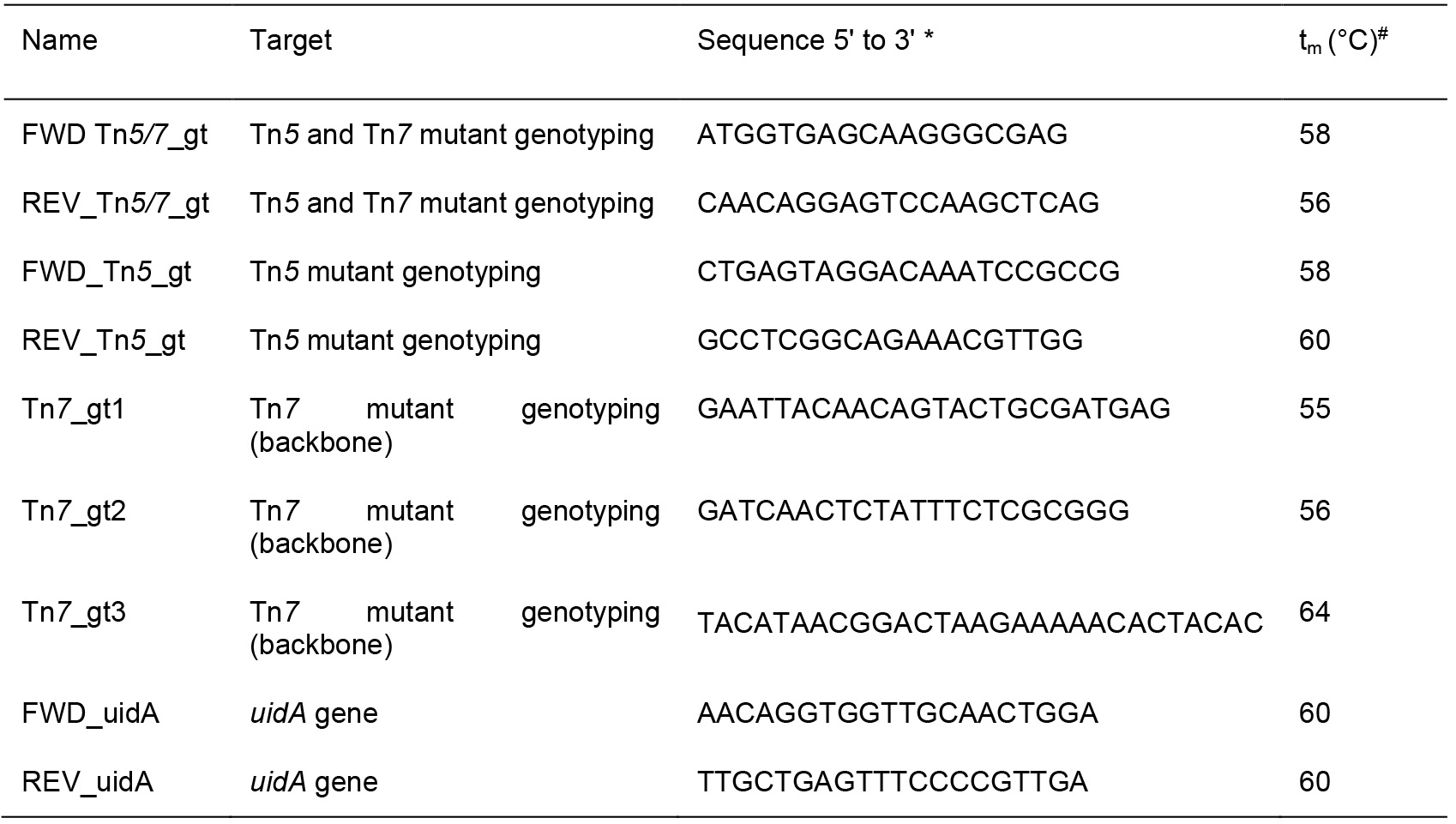
- Nucleic acid stain RedSafe (Intron, catalog number: 21141)
- Ampicillin (Duchefa Biochemie, catalog number: A0104)
- Gentamicin (Duchefa Biochemie, catalog number: G0124)
- Kanamycin (Duchefa Biochemie, catalog number: K0126)
- Chloramphenicol (Duchefa Biochemie, catalog number: C0113)
- Tetracycline (Duchefa Biochemie, catalog number: T0150)
- Ethanol (Carl Roth, catalog number: T171.5)
- K2HPO4 (Sigma, catalog number: 795496-100G)
- NaH2PO4·2H2O (Sigma, catalog number: 71505-250G)
- NH4Cl (Sigma, catalog number: A9434-500G)
- MgSO4·7H2O (Sigma, catalog number: 63138-250G)
- Na2EDTA·2H2O (Sigma, catalog number: E6635-500G)
- ZnSO4·7H2O (Sigma, catalog number: Z0251-100G)
- CoCl2·6H2O (Sigma, catalog number: C8661-25G)
- MnCl2 (Sigma, catalog number: 244589-10G)
- H3BO3 (Sigma, catalog number: 202878-10G)
- CaCl2 (Sigma, catalog number: C5670-500G)
- Na2MoO4·2H2O (Sigma, catalog number: 480967-25G)
- FeSO4·7H2O (Sigma, catalog number: F8263-500G)
- CuSO4·5H2O (Sigma, catalog number: 469130-50G)
- NaCl (Sigma, catalog number: S7653-1KG)
- KCl (Sigma, catalog number: P9333-500G)
- Na2HPO4 (Sigma, catalog number: S7907-500G)
- KH2PO4 (Sigma, catalog number: P5655-500G)
- NaOH (Sigma, catalog number: S8045-500G)
- Demineralized water
- Lysogeny broth (LB) (see Recipes)
- Lysogeny broth agar (LB agar) (see Recipes)
- Minimal medium (see Recipes)
- 10x ammonium solution
- 10x phosphate buffer solution
- 10x sulfate solution
- 1,000x trace elements solution
- 20% succinate solution
- 3% agar
- 1x Phosphate buffered saline (1x PBS) (see Recipes)
- Antibiotic stock solutions and working concentrations (see Recipes)
- Ampicillin 100 mg ml-1 stock solution
- Gentamicin 15 mg ml-1 stock solution
- Kanamycin 50 mg ml-1 stock solution
- Chloramphenicol 15 mg ml-1 stock solution
- Tetracycline 5 mg ml-1 stock solution
- TAE buffer (see Recipes)
- 0.5 M EDTA stock solution
- 50x TAE stock solution
- 1x TAE working solution
Equipment
- Forceps
- Pipettes (Eppendorf, P1000, catalog number: 3120000062; P200, catalog number: 3120000054; P10, catalog number: 3120000020)
- Erlenmeyer flasks 125 ml (e.g., Merck, catalog number: CLS4980125-12EA)
- Automatic micropipettes (Eppendorf or Gilson)
- Metal tweezers (VWR, catalog number: 232-0092)
- Bunsen burner (Eisco, catalog number: CH0087A)
- Vortex (Scientific Industries, catalog number: SI-0236)
- Autoclave (Heidolph, catalog number: 023210745CA)
- -20 °C Freezer (Fisher and Paykel, catalog number: E388LXFD1)
- Sterile bench (Labconco, catalog number: 302389100)
- Microcentrifuge for 1.5 ml centrifuge tubes with max speed > 6,000 x g (Eppendorf, catalog number: 5418000017)
- Centrifuge for 15 ml centrifuge tubes with max speed > 6,000 x g (Eppendorf, catalog number: 5805000327)
- Shaking incubators 30 °C and 37 °C (Infors, catalog number: Multitron Pro)
- Incubators 30 °C and 37 °C (Binder, catalog number: BD 56)
- Spectrophotometer (Biochrom WPA CO8000 Cell Density meter, catalog number: 80-3000-45)
- PCR thermocycler (Eppendorf, catalog number: 6311000010)
- Horizontal agarose gel electrophoresis setup including running chamber and power supply (VWR International, catalog number: 76196-448)
- Blue light or UV transilluminator (optional: camera for documentation) (Fisher Scientific, catalog number: UV95042001)
- Fluorescent microscope with appropriate filter sets for the excitation and emission spectra of the fluorescent protein tags and equipped with a 5x objective with a long working distance (Zeiss Axio Imager.M1 equipped with Objective A-Plan 5x/0.12 M27 [Zeiss, catalog number: 421030-9900-000])
Procedure
文章信息
版权信息
© 2019 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Schlechter, R. O. and Remus-Emsermann, M. N. P. (2019). Delivering "Chromatic Bacteria" Fluorescent Protein Tags to Proteobacteria Using Conjugation. Bio-protocol 9(7): e3199. DOI: 10.21769/BioProtoc.3199.
分类
微生物学 > 异源表达系统 > 非模式物种
分子生物学 > DNA > 接合
细胞生物学 > 细胞成像 > 荧光
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link