- EN - English
- CN - 中文
Measuring and Imaging the Soil-root-water System with a Light Transmission 2D Technique
利用透光二维技术对土壤-根-水系统进行测定与成像
(*contributed equally to this work) 发布: 2019年03月20日第9卷第6期 DOI: 10.21769/BioProtoc.3190 浏览次数: 8285
评审: Joëlle SchlapferKalpa MehtaKrishna Saharan
Abstract
Improving crops against water deficits requires a better understanding of plant root system functioning. This requires a better knowledge of the water uptake process and to address the influence of root system architecture or root physiological properties on the uptake efficiency. To this end, we describe here a non-destructive system that enables a dynamic, quantitative, functional imaging of the soil water and of the root system, from the single root to the whole root system scale.
This system is based on plants grown in sandy rhizotrons and relies on the modulation, by soil water content, of the intensity of light transmitted through the rhizotron. Images of the transmitted light during plant water uptake (or release) phases are recorded with a CCD camera and water content can be related to the grey level of image pixels with a calibration.
This system is affordable and can be implemented relatively easily without specific equipment. It is scalable and quick to allow the phenotyping of a range of plant genotypes relative to their water uptake pattern. This pattern can then be related with root system properties (soil colonization, root architecture) at different plant stages. Combined with modeling, imaging results help in getting parameters such as root hydraulic conductivity, distributed root water uptake rates or root xylem water potential. Combination of modeling and experiment further helps in testing biological and physiological assumptions and in predicting the uptake behavior of plants in the field.
Background
During decades, detailed observation of water uptake by plant roots, as well as water flux trough roots and plants, has been technically challenging. Most of water uptake studies have been made by measuring soil water content variation with time. In the field, such measurements are based on the use of moisture sensors distributed in soil such as neutron moisture meter (Hignett and Evett, 2002) or electromagnetic TDR or capacitive sensors (Li et al., 2002). In a controlled condition, with potted plants, the weight of the pot can be used to determine pot water content. However, if these kinds of measurements are well suited to describe macroscopic soil water behavior, they do not give access to detailed distribution of water around roots and to the interactions for water between roots. Also, they do not reveal the distribution and functioning of roots.
Concomitantly to moisture measurements in the field, root distribution can be assessed destructively with core samples, trench wall profiling or less destructively with mini-rhizotron imaging (Smit et al., 2000). This gives access to a root distribution (of length, root impacts, biovolume) with depth. The variability in rooting needs generally to acquire a high number of samples, a difficult task as sampling/measurements are highly time and labor demanding.
Plant and root hydric relationships can be examined with a variety of measuring devices. Sap flow can be estimated in stems and coarse roots with heat balance sap flow meters (Granier, 1987); root hydraulic conductance can be estimated from root segments to root systems with tension induced measurements (North and Nobel, 1995), root pressure probe (Frensch and Steudle, 1989) or pressure cell (Miyamoto et al., 2001). Root water uptake flux at the single root level can be investigated with potometer (Sanderson, 1983), dye tracing (Varney and Canny, 1993).
All these methods deliver complementary information/properties of the water-soil-plant system, but most of the time, measurements have to be conducted on excised root segments, roots or root systems separated from soil, or for plants grown in hydroponics. They also combine information on roots which might not be similar (e.g., root type, age or order, location within the root system).
Imaging techniques allow the observation of living root systems and their interaction with soil without the need of excising or extracting them from soil. They also enable to quantify root architecture and growth, root type and age if measurements are repeated in time. Ideally, imaging technique shall enable not only root imaging but also other parameters such as water content. Different imaging techniques have been used to investigate the water-soil-root system these last years. They differ in their ability to resolve roots or water, the size of samples, the 2D or 3D description, the tractability and accessibility. X-ray scanning was first used in the pioneering work of Hainsworth and Aylmore (1983 and 1986). Since then, X-ray scanners highly increased in resolution, power and scanning speed as did image processing for roots tracking and segmentation in soil columns (Tracy et al., 2010). However, accurately quantifying water and roots in 3D soils is still a challenge (Zappala et al., 2013) as the technique shows almost the same response for water and roots, with a trade-off between resolution and size of the sample (Pierret et al., 2003a and 2003b). X-ray scanners are nowadays more accessible to soil/root researchers, but still not fully accessible and rather expensive. Magnetic resonance imaging (MRI), based on the H spin relaxation in a magnetic field, has shown high potentials for imaging soil and water (Pohlmeier et al., 2008) because of its high sensitivity to water. However, the rather low resolution necessitates relatively small samples. Some natural soils cause artifacts in RMN due to their magnetic properties. A long scanning time is needed for imaging while low accessibility and high price make this technique presently not readily available for soil-root water studies. Neutron radiography (and tomography) has been recently introduced for imaging roots and water (Nakanishi, 2005; Esser et al., 2010). The technique is based on the attenuation of a thermal neutron beam and is highly sensitive to H atoms and water. It enables the imaging of roots and water at high spatial resolution (0.1-0.5 mm) and high precision of the water content (1 to 5 x 10-3 m3.m-3). The technique requires however rather thin and small root boxes (rhizotron ~30 cm). The production of a neutron beam, together with imaging capabilities, is possible only in a few places around the world and makes it hardly available for routine measurements.
A more accessible approach to study the soil-water-root system is the light transmission imaging technique, described in details in Procedure B. Originally set for studying water infiltration instability in coarse sand porous media (Glass et al., 1989), the design has been adapted to allow plant growth in an adequate sandy substrate in rhizotrons and for getting time and spatial variations of soil water. These variations can then be linked to water uptake, in relation with root colonization and root system architecture (Garrigues, 2002; Garrigues et al., 2006). Making use of only rhizotron, camera and a light source, the technique is easily scalable for phenotyping a range of root systems in relation with water uptake properties (Lobet, 2013). Coupled with modeling and complementary data, the technique can give access to plant functions/properties such as the hydraulic properties of the root system, the root growth rate affected by water content…
It shall be noticed that all of the preceding imaging methods are adapted to the scanning of samples in the laboratory. In the field, Electrical resistivity tomography has a high potential for imaging soil water content and water uptake by crops with time (Srayeddin and Doussan, 2009) but presently no method is able to simultaneously image water and the roots in the soil. Root evaluation still mostly relies on destructive sampling for field studies.
Rationale of the technique:
The technique is based on attenuation of the light passing through a rhizotron, in which roots grow and take water up (Figure 1). The light attenuation is directly linked to the water content. The technique uses easily available materials, software programs and standard CCD cameras for imaging and is cost effective. As the measurement time is short, it enables a high temporal resolution. A high spatial resolution can also be achieved, on the order of 0.2-0.5 mm. The technique pipeline can be designed to allow for multiple rhizotrons scanning and monitoring over time, in order to phenotype a range of genotypes in relation with their water uptake/root soil colonization or root architecture properties (Lobet, 2013). The measurements can be qualitative (i.e., quantifying only differences of water through time or between plants) or quantitative with a calibration step.
However, the light imaging technique has also drawbacks: (i) Plant roots grow in a thin slab of soil and is more a 2D than 3D approach. (ii) As the soil volume is rather low and soil hydric properties are those from a sandy medium, processes related to water uptake in soil (e.g., water drawdown near root, water deficit) are accelerated/amplified compared to a natural 3D soil (depending on the climatic demand). (iii) The soil in the rhizotron is specific (sand + clay) to be translucent and the technique cannot be used with a natural, opaque, soil. (iv) Bulk density of the soil is rather high, about 1.5-1.6 g/cm3, lower density results in a repacked soil which is not stable through time. At least for maize, lupine, Arabidopsis the bulk density and soil type did not impede/alter root growth.
To date, most applications with light transmission imaging have been water shortage experiments and analyze the water uptake pattern in relation with root distribution/architecture or root hydraulics (Figure 2). However, this technique can be used to examine water uptake as a function of age/plant stage; to study interaction/competition between plant neighbors; to follow the effects with time of humectation/drying cycles; the interaction between localized irrigation and root growth/water uptake; split-roots or partial root zone drying can also be explored as well as root growth for various heterogeneous water content conditions or induction/regulation of aquaporins. Complementary data, such as leaf water potential, transpiration of leaves, or other physiological measurements can be of interest to examine water relations at the plant level.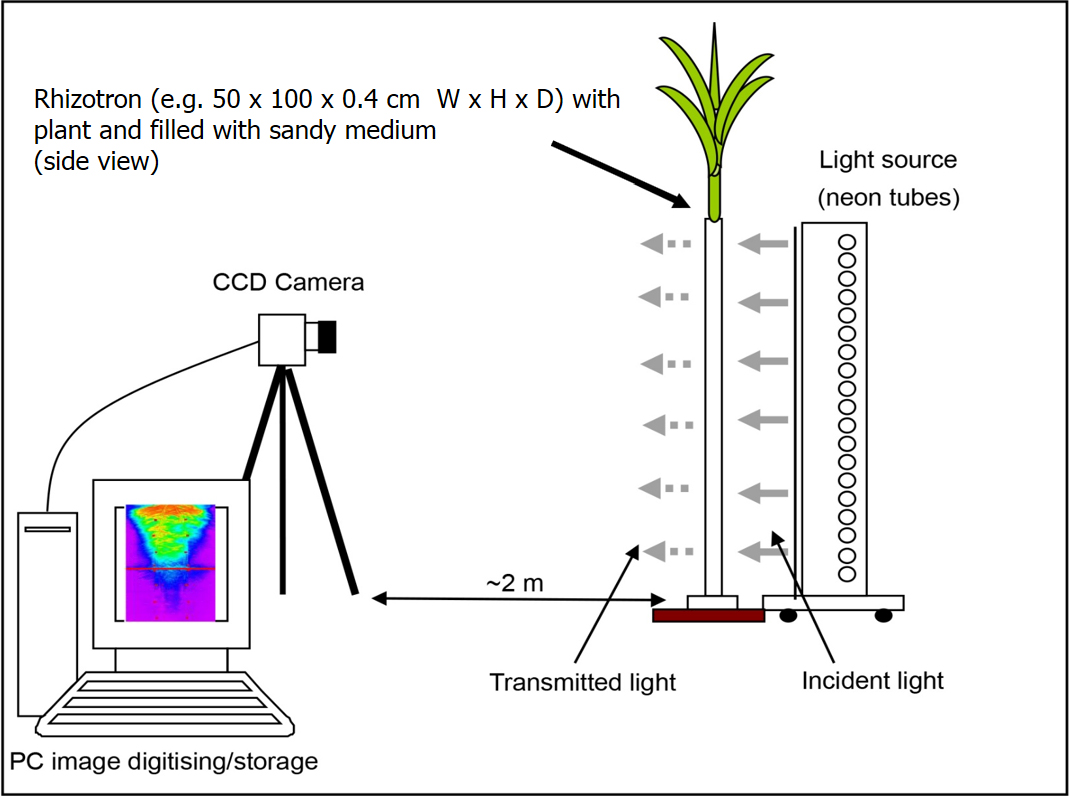
Figure 1. Schematic layout of the experimental system for imaging roots and soil water content (reprint from Garrigues et al., 2006 with permission from Plant and Soil)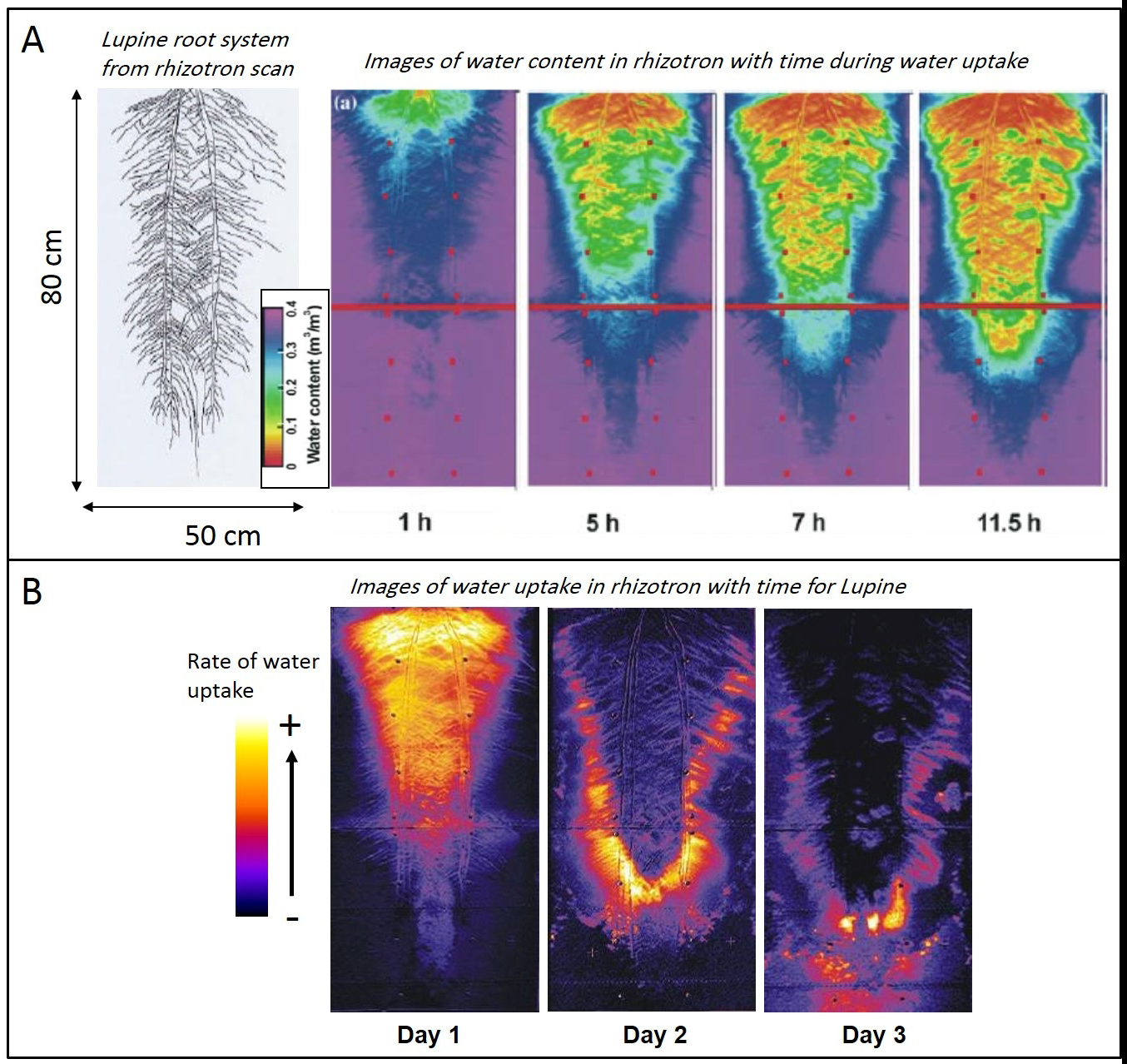
Figure 2. Examples of results from Light transmission imaging of the soil-root system. A. Variation with time (after the end of irrigation) of the water content in the soil of the rhizotron. The soil is colonized by a 50-days old Lupine whose root system is shown on the left. B. Water uptake rates of the Lupine in the rhizotron, derived from the difference between images of water content (shown in A) in the morning and evening. A downward and lateral spreading of uptake is visible. This spatial-temporal pattern depends on root architecture, root hydraulic conductance distribution in the root system and soil hydric properties. (Panel A is adapted from Doussan et al. 2006)
Theoretical background:
The principle of the technique relies on the fact that the light transmitted through a sandy porous medium (sand + air + water) increases with the water content. This variation in transmitted light intensity with water content is linked to the physical processes of reflection/refraction of light. Indeed, the light passing through the different phases of the porous medium is not only exponentially absorbed, but also reflected and refracted at the interfaces between the phases. For the later processes, the transmitted intensity of the passing light is a function of the refractive index of the two phases and angle incidence. The Fresnel’s law gives the light transmission ratio (![]() ; with Iv: intensity of transmitted light, Ii: intensity of the incident light) at the interface of two media for a normal incidence:
; with Iv: intensity of transmitted light, Ii: intensity of the incident light) at the interface of two media for a normal incidence: 
where n is the ratio of the refractive indices of the two phases. When using the refractive index of sand, water and air (1.6, 1.33, 1.0 respectively), the transmission ratio for the sand–air ![]() interface is 0.946 while for sand–water interface
interface is 0.946 while for sand–water interface ![]() it reaches 0.991 (Tidwell and Glass, 1994). Hence, when water replaces air at sand interfaces, the transmitted light increases.
it reaches 0.991 (Tidwell and Glass, 1994). Hence, when water replaces air at sand interfaces, the transmitted light increases.
Tidewell and Glass (1994) generalized equation (1) to a whole porous medium, assuming that a pore is full or empty of water: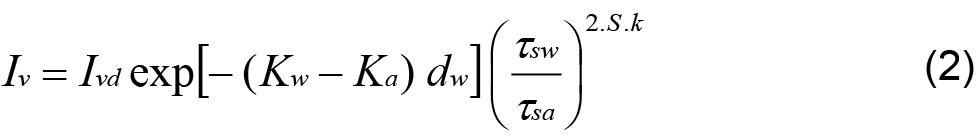
where Ivd is emergent light intensity of the dry sample, Kw and Ka are the light absorption coefficient for water and air, respectively; dw is the total thickness of water filled pores, S is the water saturation: ![]() with
with ![]() volumetric water content and
volumetric water content and 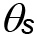 water content at saturation), k is the average number of pores across the sample.
water content at saturation), k is the average number of pores across the sample.
As Kw and Ka nearly equal, eq. (2) can be approximated by: 
As the dry state for estimating Ivd is not readily attainable after plant growth in the medium and (3) is dependent on the bulk density of the sample, we can make use of the saturated state for normalization between samples. Using the ratio ![]() , where
, where 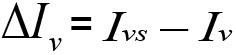 , with Ivs the emergent light intensity at water saturation, equation (3) can be transformed to:
, with Ivs the emergent light intensity at water saturation, equation (3) can be transformed to: 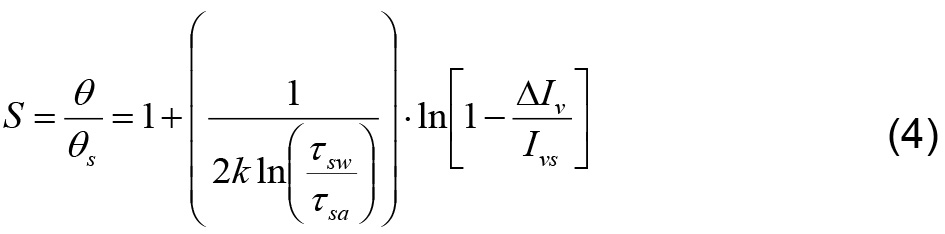
Based on (4), fitting a relationship between S and ln(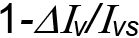 ) is the rational for calibration and the conversion of images’ pixel intensity into water content.
) is the rational for calibration and the conversion of images’ pixel intensity into water content.
Materials and Reagents
- Growing media for plant
- Filter paper
- Petri dishes
- Sand: “Fontainebleau sand” (VWR, catalog number: 27460.364, 5kg)
- Clay: hectorite (Elementis specialties: Bentone MA®)
- Coarse sand/gravel (2-3 mm diameter, such aquarium sand)
- Detergent: TFD4 (Dutscher, catalog number: 711169B)
- Nutrient solution: modified Hoagland solution (see Recipes, chemicals available from VWR, Fisher Scientific, Sigma-Aldrich, …) made with:
- KNO3
- Ca(NO3)2·4H2O
- Fe-EDTA
- MgSO4·7H2O
- NH4NO3
- KH2PO4
- H3BO3
- MnCl2·4H2O
- ZnSO4·7H2O
- CuSO4
- H3MoO4 or Na2MoO4·2H2O
- KOH
- Construction of Rhizotrons (height x width from 30 x 30 to 100 x 50 cm)
- PMMA (Plexiglas/Perspex) sheets: Transparent and colourless, 5 mm thick (minimum)
- PVC rod: 1 cm high, 4 mm thick
- Aluminum flat bars: 4 mm thick, 1 cm width (see Notes)
- Corner shaped aluminum bar, 1 x 1 cm
- Stainless steel and nylon bolts (and nuts): 20 mm long x 3 mm diameter
- Black matt tape
- Plastics (PVC, PPMA, Altuglass) (is easily available from local retailers, e.g., Gaches Chimie, abaqueplast: abaqueplast.fr)
- Aluminum bars (available from local DIY stores)
- Construction of Light source and colorimetric scale (if needed)
- Fluorescent tubes (e.g., Philips TLD 18W/840–Cool white color)
- Wood or other materials for constructing the frame of the light illuminating box
- Light diffusing sheet of PMMA: 4 mm thick, white colored (Altuglass, catalog number: 100 27018)
Equipment
- CCD Camera: 6 to 12 Mpixels, 8 to 14 bit depth for colors, RAW format available, computer driven interface for remotely shooting the picture and transferring the image file, manual adjustment possible
- Camera lens: 50 to 120 mm focal length, aperture ~f2-f22
- Tripod for camera
- Balance for weighing rhizotrons (20 kg range for the larger 1 x 0.5 m rhizotron)
Software
- Image processing Software: Fiji (Schindelin et al., 2012, open-source software based on ImageJ, https://imagej.net/Fiji)
- For processing imaged root systems: SmartRoot (Lobet et al., 2011, Open-source, based on ImageJ, https://smartroot.github.io/) or for simple segmentation of root system (and length, diameter of roots): IJ_Rhizo (Pierret et al., 2013, Open-source, based on ImageJ, http://www.plant-image-analysis.org/software/IJ_Rhizo)
Procedure
文章信息
版权信息
© 2019 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Doussan, C. and Garrigues, E. (2019). Measuring and Imaging the Soil-root-water System with a Light Transmission 2D Technique. Bio-protocol 9(6): e3190. DOI: 10.21769/BioProtoc.3190.
分类
植物科学 > 植物生理学 > 水转运
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link











