- EN - English
- CN - 中文
Isolation of Neural Stem Cells from the Embryonic Mouse Hippocampus for in vitro Growth or Engraftment into a Host Tissue
从胚胎小鼠海马分离神经干细胞用于体外生长或植入宿主组织
发布: 2019年02月20日第9卷第4期 DOI: 10.21769/BioProtoc.3165 浏览次数: 10067
评审: Xi FengNolwenn PasquetKhyati Hitesh Shah
Abstract
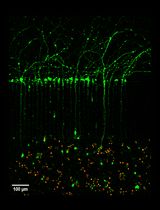
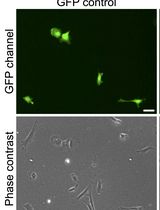
For both stem cell research and treatment of the central nervous system disorders, neural stem/progenitor cells (NSPCs) represent an important breakthrough tool. In the expanded stem cell-based therapy use, NSPCs not only provide a powerful cell source for neural cell replacement but a useful model for developmental biology research. Despite numerous approaches were described for isolation of NSPCs from either fetal or adult brain, the main issue remains in extending cell survival following isolation. Here we provide a simple and affordable protocol for making viable NSPCs from the fetal mouse hippocampi, which are capable of maintaining the high viability in a 2D monolayer cell culture or generating 3D neuro-spheroids of cell aggregates. Further, we describe the detailed method for engraftment of embryonic NSPCs onto a host hippocampal tissue for promoting multilinear cell differentiation and maturation within endogenous environment. Our experimental data demonstrate that embryonic NSPCs isolated using this approach show the high viability (above 88%). Within a host tissue, these cells were capable of differentiating to the main neural subpopulations (principal neurons, oligodendrocytes, astroglia). Finally, NSPC-derived neurons demonstrated matured functional properties (electrophysiological activity), becoming functionally integrated into the host hippocampal circuits within a couple of weeks after engraftment.
Keywords: Embryonic neural stem/progenitor cells (NSPCs) (胚胎神经干/祖细胞)Background
Multipotent stem cells of neural origin, neural stem/progenitor cells (NSPCs), represent a versatile tool for enabling nerve cell replacement in a number of neurological disorders characterized by the loss of specialized neural subpopulations. The unlimited lifespan and directed NSPC differentiation into the key neural subtypes (neurons, astrocytes, and oligodendrocytes) make these cells the most attractive candidate for emerging stem cell-based therapies for presently incurable brain disorders (Kopach and Pivneva, 2018). Stem cells exist in the developing and mature nervous system and can be isolated from either fetal brain (embryonic NSPCs) or, as established more recently, from mature brain (in adults, both subventricular and subgranular zones in dentate gyrus of the hippocampus, forebrain, cerebellum and olfactory bulb) (Pagano et al., 2000; Roy et al., 2000; Klein et al., 2005; Behnan et al., 2017; Kempermann et al., 2018). Despite that adult stem cells bear expectations for an advanced translational approach (Kempermann et al., 2018), a limited number of rigorous evidence supports this research avenue at the moment.
Accumulating evidence for the properties of fetal NSPCs and phenotype specifications, confirmed by dedicated clinical trials, retains a focus on this stem cell type. Furthermore, embryonic NSPCs represent an essential tool for developmental biology research. Various methodological approaches were probed to isolate NSPCs from the fetal brains and to maintain cells in conditions determining their high viability, hence, progeny. Such conditions largely dictate phenotyping of differentiating progenitors to acquire the competitive NSPC-derived cells. The two main strategies for preserving the high viability of isolated NSPCs exist in i) cell culture in vitro and ii) grafting into a host tissue. A classical 2D culture, an adherently expanded monolayer of cells, has been routinely used for stem cell growth in vitro and/or for directed phenotypic differentiation (Conti et al., 2005; Pollard et al., 2006). In parallel, newer developed approach of generating 3D cultures, neuro-spheroids of an assembly of NSPCs at various phenotypic and developmental stages, has been emerged to represent more physiological conditions pertinent to neuronal differentiation rather than glial (Ignatova et al., 2002; Suslov et al., 2002; Makri et al., 2010). This approach can potentially enhance the therapeutic potential of NSPCs in vitro. Another alternative–grafting of viable NSPCs into brain tissue–fulfill a strategy for promoting the multi-lineage differentiation of progenitors within a host tissue, regulated by endogenous environment. Such an approach has recently been implemented in the treatment of the ischemia-damaged hippocampal tissue (Tsupykov et al., 2014; Kopach et al., 2018).
Here we provide the detailed protocol that we routinely use for isolation of viable NSPCs from the fetal mouse hippocampus. This protocol was also utilized for engraftment of undifferentiated cells to the hippocampal tissue for monitoring maturation of NSPC-derived cells within a host environment in our previous studies (Kopach et al., 2018). We describe all the details on how to test the viability of the obtained embryonic NSPCs and maintain the cells in multiple approaches for further proposed use.
Materials and Reagents
- Materials
- 15 ml and 50 ml centrifuge tubes (Corning, catalog numbers: 430790, 430828)
- 1.5 ml Eppendorf tubes (Sigma-Aldrich, catalog number: T9661)
- Glass Pasteur pipette (1 mm diameter) (Fisher Scientific, catalog number: 13-678-6A)
- 35 mm, 60 mm, and 100 mm tissue culture dishes (CELLSTAR, catalog numbers: 627160, 628160, 664160)
- Falcon® 40-µm cell strainer (Corning, Falcon®, catalog number: 352340)
- 0.45-µm sterile filter (Fisher Scientific, MerckTM, catalog number: 12279299)
- 6-well and 24-well tissue culture plates (Greiner Bio-One, catalog numbers: 657160, 662160)
- 13 mm glass coverslips (Henz Herenz, catalog number: 1051204)
- SuperFrost® glass slides (VWR, Thermo Fisher Scientific, catalog number: 631-0706)
- Plastic serological pipettes (5 ml, 10 ml, and 25 ml) (Corning® Costar® Stripette®, catalog numbers: 4487, 4488, 4489)
- Pipette tips (TipOne, STARLAB, catalog numbers: S1111-3700, S1111-1706, S1111-6701)
- Millipore® Millicell® cell culture plate inserts (Sigma-Aldrich, catalog number: Z353086)
- Whatman® qualitative filter paper (Sigma-Aldrich, catalog number: WHA1001110)
- Scalpel blades (Fisher Scientific, Swann-MortonTM, catalog number: 11772724)
- Scalpel with retractable blade (Eickemeyer®, catalog number: 100504)
- Parafilm® M (Sigma-Aldrich, catalog number: P7793)
- Animals
- Pregnant FVB-Cg-Tg(GFPU) 5Nagy/J GFP mice (obtained from the animal facilities of State Institute of Genetic and Regenerative Medicine)
- Embryos of FVB-Cg-Tg(GFPU) 5Nagy/J GFP mice at the developmental stage of E16-E17
- Pups of FVB mice (8 to 9-days old)
- Reagents
- HBBS without calcium and magnesium (Sigma-Aldrich, catalog number: 55021C)
- Paraformaldehyde (PFA, Sigma-Aldrich, catalog number: P6148)
- 0.25% trypsin-EDTA (Sigma-Aldrich, catalog number: T4049)
- 22% Percoll (Sigma-Aldrich, catalog number: 17-0891-01)
- Neurobasal® medium (Thermo Fisher Scientific, GibcoTM, catalog number: 21103049)
- B-27® supplement (50x) (Thermo Fisher Scientific, GibcoTM, catalog number: 17504044)
- GlutaMAXTM supplement (100x) (Thermo Fisher Scientific, GibcoTM, catalog number: 35050061)
- N-acetyl-L-cysteine (NAC) (Sigma-Aldrich, catalog number: A7250)
- Penicillin-streptomycin (P/S) solution (100,000 U/ml) (Thermo Fisher Scientific, GibcoTM, catalog number: 15140122)
- FGF-2 (Sigma-Aldrich, catalog number: SRP4037)
- Matrigel (BD Biosciences, catalog number: 354234)
- Triton® X-100 (Sigma-Aldrich, catalog number: T8787)
- Accutase® solution (Sigma-Aldrich, catalog number: A6964)
- 0.4% Trypan blue (Thermo Fisher Scientific, GibcoTM, catalog number: 15250061)
- Antibodies
- Primary
- Mouse anti-Nestin antibody (1:100) (Santa Cruz Biotechnology, catalog number: sc-23927)
- Rabbit anti-β-tubulin III (1:500) (Sigma-Aldrich, catalog number: T2200)
- Mouse anti-GFAP (1:200) (Sigma-Aldrich, catalog number: AMAB91033)
- Rabbit anti-olig-2 (1:200) (Abcam, catalog number: ab136253)
- Mouse anti-NeuN (1:100) (Millipore, catalog number: MAB377)
- Secondary
- Donkey anti-mouse Alexa Fluor 555 (1:1,000) (Thermo Fisher Scientific, catalog number: A-31570)
- Donkey anti-rabbit Alexa Fluor 647 (1:1,000) (Thermo Fisher Scientific, catalog number: A-31573)
- Nuclei tracer Hoechst 33342 (1:5,000) (Sigma-Aldrich, catalog number: 14533)
- Primary
- ImmunoHistoMountTM (Sigma-Aldrich, catalog number: I1161)
- 70% Ethanol (Sigma-Aldrich, catalog number: 459836)
- Sodium dihydrogen phosphate monobasic (NaH2PO4) (Sigma-Aldrich, catalog number: S0751)
- Disodium phosphate dibasic (Na2HPO4) (Sigma-Aldrich, catalog number: S0876)
- Sodium hydroxide (NaOH) (Sigma-Aldrich, catalog number: S8045)
- Minimum Essential Medium (MEM) (Sigma-Aldrich, catalog number: M2249)
- Tris (Sigma-Aldrich, catalog number: 252859)
- Sodium bicarbonate (Sigma-Aldrich, catalog number: 792519)
- HEPES (Thermo Fisher Scientific, catalog number: 15630080)
- D(+)-glucose (Sigma-Aldrich, catalog number: G8270)
- Horse serum (Sigma-Aldrich, catalog number: H1138)
- Phosphate buffered saline (PBS), pH 7.4 (Sigma-Aldrich, catalog number: P3813)
- BD Cell Wash buffer (BD Biosciences, catalog number: 349524)
- Bovine serum albumin (BSA) (Sigma-Aldrich, catalog number: 05470)
- 7-AAD (BD PharmingenTM, catalog number: 559925)
- 0.2 M Phosphate buffer (pH 7.4) (see Recipes)
- 4% Paraformaldehyde (PFA) in 0.1 M phosphate buffer (see Recipes)
- Growth medium (see Recipes)
- Tissue dissecting medium (pH 7.3) (see Recipes)
- Tissue culture medium (pH 7.2) (see Recipes)
- Antibody solution (see Recipes)
- Blocking solution (see Recipes)
Equipment
- Pipettes (Eppendorf, Research® Plus, catalog numbers: EP 3123000012; EP 3123000098; EP 3123000055; EP 3120000062)
- 250 ml Graduated cylinder (Karter Scientific, catalog number: 213I13)
- Dry heat sterilizing cabinet (Grande, model: GIO-072)
- Fine tools for dissection and manipulating embryonic brains
- Dissecting scissors (Dumont, catalog number: 14090-09)
- Small spatula (Dumont, catalog number: 10090-13)
- Forceps (Dumont, catalog number: 91197-00)
- Angled forceps (Dumont, catalog number: 00125-11)
- C-Chip disposable hemocytometer/Neubauer (Labtech International, Heathfield, UK, catalog number: DHC-N01)
- Refrigerated centrifuge (Thermo Fisher Scientific, model: IEC-CL30R)
- Water bath (Biosan, model: WB-4MS)
- Biosafety Cabinet Class II Type A2 (Labconco, model: Purifier Logic)
- CO2 incubator (Thermo Fisher Scientific, model: SteriCycle 371)
- Flow cytometer (Becton Dickinson, model: BD FACSAria сell sorter)
- Tissue chopper (McIlwain, model: MTC/2E)
- Alcohol burner (Sigma-Aldrich, catalog number: Z509604)
- Inverted microscope (Olympus, model: IX71, equipped with camera DP-20)
- Confocal microscope (Olympus, model: FV1000)
- Stereo microscope (Olympus, model: SZX7)
- 4 °C refrigerator (Haier Biomedical, model: HYC-940)
Software
- QuickPHOTO software (PROMICRA, s.r.o., Czech Republic)
- FV10-ASW software (Olympus, Japan)
- BD FACSDivaTM 6.1.2 software (BD Biosciences, USA)
Procedure
文章信息
版权信息
© 2019 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Rybachuk, O., Kopach, O., Pivneva, T. and Kyryk, V. (2019). Isolation of Neural Stem Cells from the Embryonic Mouse Hippocampus for in vitro Growth or Engraftment into a Host Tissue. Bio-protocol 9(4): e3165. DOI: 10.21769/BioProtoc.3165.
分类
干细胞 > 胚胎干细胞 > 维持和分化
神经科学 > 细胞机理 > 组织分离与培养
细胞生物学 > 细胞活力 > 细胞死亡
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link